LPS- or Pseudomonas aeruginosa-mediated activation of the macrophage TLR4 signaling cascade depends on membrane lipid composition
- PMID: 26870615
- PMCID: PMC4748739
- DOI: 10.7717/peerj.1663
LPS- or Pseudomonas aeruginosa-mediated activation of the macrophage TLR4 signaling cascade depends on membrane lipid composition
Abstract
It is well known that PUFA impede the LPS-mediated activation of the transcription factor NFkappaB. However, the underlying mode of action has not been clarified yet. To address this issue in a comprehensive approach, we used the monocyte/macrophage cell line RAW264.7 to investigate the consequences of a PUFA supplementation on the TLR4 pathway with a focus on (i) the gene expression of TLR4 itself as well as of its downstream mediators, (ii) the membrane microdomain localization of TLR4 and CD14, (iii) the stimulation-induced interaction of TLR4 and CD14. Our data indicate that the impairment of the TLR4-mediated cell activation by PUFA supplementation is not due to changes in gene expression of mediator proteins of the signaling cascade. Rather, our data provide evidence that the PUFA enrichment of macrophages affects the TLR4 pathway at the membrane level. PUFA incorporation into membrane lipids induces a reordering of membrane microdomains thereby affecting cellular signal transduction. It is important to note that this remodeling of macrophage rafts has no adverse effect on cell viability. Hence, microdomain disruption via macrophage PUFA supplementation has a potential as non-toxic strategy to attenuate inflammatory signaling.
Keywords: CD14; Lipid rafts; Macrophages; PUFA; TLR4.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
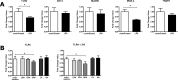
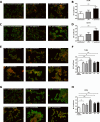
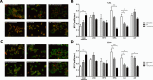
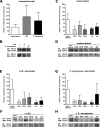
Similar articles
-
Lipid raft localization of TLR2 and its co-receptors is independent of membrane lipid composition.PeerJ. 2018 Jan 5;6:e4212. doi: 10.7717/peerj.4212. eCollection 2018. PeerJ. 2018. PMID: 29312832 Free PMC article.
-
The prohibitin complex regulates macrophage fatty acid composition, plasma membrane packing, and lipid raft-mediated inflammatory signaling.Prostaglandins Leukot Essent Fatty Acids. 2023 Mar;190:102540. doi: 10.1016/j.plefa.2023.102540. Epub 2023 Jan 19. Prostaglandins Leukot Essent Fatty Acids. 2023. PMID: 36706677 Free PMC article.
-
Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury.J Neuroinflammation. 2017 Jul 24;14(1):143. doi: 10.1186/s12974-017-0917-3. J Neuroinflammation. 2017. PMID: 28738820 Free PMC article.
-
Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts.Alcohol Clin Exp Res. 2006 Jan;30(1):76-85. doi: 10.1111/j.1530-0277.2006.00003.x. Alcohol Clin Exp Res. 2006. PMID: 16433734
-
Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling.Cell Mol Life Sci. 2015 Feb;72(3):557-581. doi: 10.1007/s00018-014-1762-5. Epub 2014 Oct 22. Cell Mol Life Sci. 2015. PMID: 25332099 Free PMC article. Review.
Cited by
-
Glycyrrhizin Interacts with TLR4 and TLR9 to Resolve P. aeruginosa Keratitis.Pathogens. 2022 Nov 11;11(11):1327. doi: 10.3390/pathogens11111327. Pathogens. 2022. PMID: 36422579 Free PMC article.
-
Saturated Fatty Acids in Obesity-Associated Inflammation.J Inflamm Res. 2020 Jan 6;13:1-14. doi: 10.2147/JIR.S229691. eCollection 2020. J Inflamm Res. 2020. PMID: 32021375 Free PMC article. Review.
-
The Regulation of Neutrophil Migration in Patients with Sepsis: The Complexity of the Molecular Mechanisms and Their Modulation in Sepsis and the Heterogeneity of Sepsis Patients.Cells. 2023 Mar 24;12(7):1003. doi: 10.3390/cells12071003. Cells. 2023. PMID: 37048076 Free PMC article. Review.
-
Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications?Pharmacol Ther. 2021 Mar;219:107703. doi: 10.1016/j.pharmthera.2020.107703. Epub 2020 Oct 5. Pharmacol Ther. 2021. PMID: 33031856 Free PMC article. Review.
-
Isomeric lipid signatures reveal compartmentalized fatty acid metabolism in cancer.J Lipid Res. 2022 Jun;63(6):100223. doi: 10.1016/j.jlr.2022.100223. Epub 2022 May 7. J Lipid Res. 2022. PMID: 35537528 Free PMC article.
References
-
- Adolph S, Schoeniger A, Fuhrmann H, Schumann J. Unsaturated fatty acids as modulators of macrophage respiratory burst in the immune response against Rhodococcus equi and Pseudomonas aeruginosa. Free Radical Biology & Medicine. 2012;52(11-12):2246–2253. doi: 10.1016/j.freeradbiomed.2012.04.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

