Multisite tyrosine phosphorylation of the N-terminus of Mint1/X11α by Src kinase regulates the trafficking of amyloid precursor protein
- PMID: 26865271
- PMCID: PMC4982022
- DOI: 10.1111/jnc.13571
Multisite tyrosine phosphorylation of the N-terminus of Mint1/X11α by Src kinase regulates the trafficking of amyloid precursor protein
Abstract
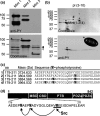
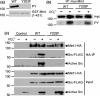
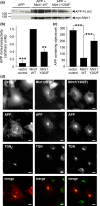
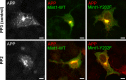
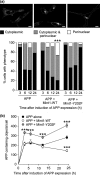
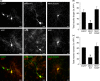
Mint/X11 is one of the four neuronal trafficking adaptors that interact with amyloid precursor protein (APP) and are linked with its cleavage to generate β-amyloid peptide, a key player in the pathology of Alzheimer's disease. How APP switches between adaptors at different stages of the secretory pathway is poorly understood. Here, we show that tyrosine phosphorylation of Mint1 regulates the destination of APP. A canonical SH2-binding motif ((202) YEEI) was identified in the N-terminus of Mint1 that is phosphorylated on tyrosine by C-Src and recruits the active kinase for sequential phosphorylation of further tyrosines (Y191 and Y187). A single Y202F mutation in the Mint1 N-terminus inhibits C-Src binding and tyrosine phosphorylation. Previous studies observed that co-expression of wild-type Mint1 and APP causes accumulation of APP in the trans-Golgi. Unphosphorylatable Mint1 (Y202F) or pharmacological inhibition of Src reduced the accumulation of APP in the trans-Golgi of heterologous cells. A similar result was observed in cultured rat hippocampal neurons where Mint1(Y202F) permitted the trafficking of APP to more distal neurites than the wild-type protein. These data underline the importance of the tyrosine phosphorylation of Mint1 as a critical switch for determining the destination of APP. The regulation of amyloid precursor protein (APP) trafficking is poorly understood. We have discovered that the APP adapter, Mint1, is phosphorylated by C-Src kinase. Mint1 causes APP accumulation in the trans-Golgi network, whereas inhibition of Src or mutation of Mint1-Y202 permits APP recycling. The phosphorylation status of Mint1 could impact on the pathological trafficking of APP in Alzheimer's disease.
Keywords: Mint1; Src; amyloid precursor protein; intracellular trafficking; protein phosphorylation; tyrosine kinase.
© 2016 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures


Similar articles
-
Intracellular amyloid precursor protein sorting and amyloid-β secretion are regulated by Src-mediated phosphorylation of Mint2.J Neurosci. 2012 Jul 11;32(28):9613-25. doi: 10.1523/JNEUROSCI.0602-12.2012. J Neurosci. 2012. PMID: 22787047 Free PMC article.
-
Mint proteins are required for synaptic activity-dependent amyloid precursor protein (APP) trafficking and amyloid β generation.J Biol Chem. 2014 May 30;289(22):15374-83. doi: 10.1074/jbc.M113.541003. Epub 2014 Apr 17. J Biol Chem. 2014. PMID: 24742670 Free PMC article.
-
A new Mint1 isoform, but not the conventional Mint1, interacts with the small GTPase Rab6.PLoS One. 2013 May 30;8(5):e64149. doi: 10.1371/journal.pone.0064149. Print 2013. PLoS One. 2013. PMID: 23737971 Free PMC article.
-
The amyloid precursor protein and its network of interacting proteins: physiological and pathological implications.Brain Res Brain Res Rev. 2005 Apr;48(2):257-64. doi: 10.1016/j.brainresrev.2004.12.016. Brain Res Brain Res Rev. 2005. PMID: 15850665 Review.
-
The X11/Mint family of adaptor proteins.Brain Res Rev. 2006 Sep;52(2):305-15. doi: 10.1016/j.brainresrev.2006.04.005. Epub 2006 Jun 9. Brain Res Rev. 2006. PMID: 16764936 Review.
Cited by
-
Quintessential Synergy: Concurrent Transient Administration of Integrated Stress Response Inhibitors and BACE1 and/or BACE2 Activators as the Optimal Therapeutic Strategy for Alzheimer's Disease.Int J Mol Sci. 2024 Sep 13;25(18):9913. doi: 10.3390/ijms25189913. Int J Mol Sci. 2024. PMID: 39337400 Free PMC article.
-
Advances in the cell biology of the trafficking and processing of amyloid precursor protein: impact of familial Alzheimer's disease mutations.Biochem J. 2024 Oct 2;481(19):1297-1325. doi: 10.1042/BCJ20240056. Biochem J. 2024. PMID: 39302110 Free PMC article. Review.
-
Effects of the Pentapeptide P33 on Memory and Synaptic Plasticity in APP/PS1 Transgenic Mice: A Novel Mechanism Presenting the Protein Fe65 as a Target.Int J Mol Sci. 2019 Jun 22;20(12):3050. doi: 10.3390/ijms20123050. Int J Mol Sci. 2019. PMID: 31234498 Free PMC article.
-
On the Inadequacy of the Current Transgenic Animal Models of Alzheimer's Disease: The Path Forward.Int J Mol Sci. 2024 Mar 4;25(5):2981. doi: 10.3390/ijms25052981. Int J Mol Sci. 2024. PMID: 38474228 Free PMC article.
-
Attenuation of amyloid-β generation by atypical protein kinase C-mediated phosphorylation of engulfment adaptor PTB domain containing 1 threonine 35.FASEB J. 2019 Nov;33(11):12019-12035. doi: 10.1096/fj.201802825RR. Epub 2019 Aug 5. FASEB J. 2019. PMID: 31373844 Free PMC article.
References
-
- Abramoff M. D., Magelhaes P. J. and Ram S. J. (2004) Image Processing with ImageJ. Biophotonics Int 11, 36–42.
-
- Ando K., Iijima K. I., Elliott J. I., Kirino Y. and Suzuki T. (2001) Phosphorylation‐dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta‐amyloid. J. Biol. Chem. 276, 40353–40361. - PubMed
-
- Belfield J. L., Whittaker C., Cader M. Z. and Chawla S. (2006) Differential effects of Ca2 + and cAMP on transcription mediated by MEF2D and cAMP‐response element‐binding protein in hippocampal neurons. J. Biol. Chem. 281, 27724–27732. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

