Ethanol Production by Selected Intestinal Microorganisms and Lactic Acid Bacteria Growing under Different Nutritional Conditions
- PMID: 26858714
- PMCID: PMC4732544
- DOI: 10.3389/fmicb.2016.00047
Ethanol Production by Selected Intestinal Microorganisms and Lactic Acid Bacteria Growing under Different Nutritional Conditions
Abstract
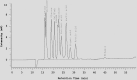
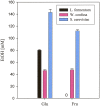
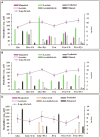
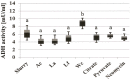
To gain some specific insight into the roles microorganisms might play in non-alcoholic fatty liver disease (NAFLD), some intestinal and lactic acid bacteria and one yeast (Anaerostipes caccae, Bacteroides thetaiotaomicron, Bifidobacterium longum, Enterococcus fecalis, Escherichia coli, Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus plantarum, Weissella confusa, Saccharomyces cerevisiae) were characterized by high performance liquid chromatography for production of ethanol when grown on different carbohydrates: hexoses (glucose and fructose), pentoses (arabinose and ribose), disaccharides (lactose and lactulose), and inulin. Highest amounts of ethanol were produced by S. cerevisiae, L. fermentum, and W. confusa on glucose and by S. cerevisiae and W. confusa on fructose. Due to mannitol-dehydrogenase expressed in L. fermentum, ethanol production on fructose was significantly (P < 0.05) reduced. Pyruvate and citrate, two potential electron acceptors for regeneration of NAD(+)/NADP(+), drastically reduced ethanol production with acetate produced instead in L. fermentum grown on glucose and W. confusa grown on glucose and fructose, respectively. In fecal slurries prepared from feces of four overweight volunteers, ethanol was found to be produced upon addition of fructose. Addition of A. caccae, L. acidophilus, L. fermentum, as well as citrate and pyruvate, respectively, abolished ethanol production. However, addition of W. confusa resulted in significantly (P < 0.05) increased production of ethanol. These results indicate that microorganisms like W. confusa, a hetero-fermentative, mannitol-dehydrogenase negative lactic acid bacterium, may promote NAFLD through ethanol produced from sugar fermentation, while other intestinal bacteria and homo- and hetero-fermentative but mannitol-dehydrogenase positive lactic acid bacteria may not promote NAFLD. Also, our studies indicate that dietary factors interfering with gastrointestinal microbiota and microbial metabolism may be important in preventing or promoting NAFLD.
Keywords: Weissella confusa; arabinose; ethanol; fecal slurries; fructose; inulin; lactulose; non-alcoholic fatty liver disease.
Figures
References
-
- Amaretti A., Bernardi T., Tamburini E., Zanoni S., Lomma M., Matteuzzi D., et al. . (2007). Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Appl. Environ. Microbiol. 73, 3637–3644. 10.1128/AEM.02914-06 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

