Activation of RNase L is dependent on OAS3 expression during infection with diverse human viruses
- PMID: 26858407
- PMCID: PMC4776461
- DOI: 10.1073/pnas.1519657113
Activation of RNase L is dependent on OAS3 expression during infection with diverse human viruses
Erratum in
-
Correction for Li et al., Activation of RNase L is dependent on OAS3 expression during infection with diverse human viruses.Proc Natl Acad Sci U S A. 2019 Jun 18;116(25):12573. doi: 10.1073/pnas.1908765116. Epub 2019 Jun 10. Proc Natl Acad Sci U S A. 2019. PMID: 31182583 Free PMC article. No abstract available.
Abstract
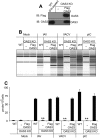
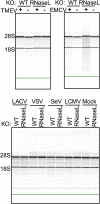
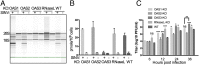
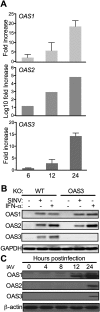
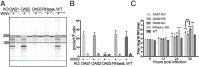
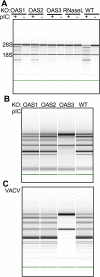
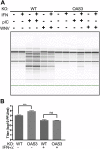
The 2',5'-oligoadenylate (2-5A) synthetase (OAS)-RNase L system is an IFN-induced antiviral pathway. RNase L activity depends on 2-5A, synthesized by OAS. Although all three enzymatically active OAS proteins in humans--OAS1, OAS2, and OAS3--synthesize 2-5A upon binding dsRNA, it is unclear which are responsible for RNase L activation during viral infection. We used clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein-9 nuclease (Cas9) technology to engineer human A549-derived cell lines in which each of the OAS genes or RNase L is knocked out. Upon transfection with poly(rI):poly(rC), a synthetic surrogate for viral dsRNA, or infection with each of four viruses from different groups (West Nile virus, Sindbis virus, influenza virus, or vaccinia virus), OAS1-KO and OAS2-KO cells synthesized amounts of 2-5A similar to those synthesized in parental wild-type cells, causing RNase L activation as assessed by rRNA degradation. In contrast, OAS3-KO cells synthesized minimal 2-5A, and rRNA remained intact, similar to infected RNase L-KO cells. All four viruses replicated to higher titers in OAS3-KO or RNase L-KO A549 cells than in parental, OAS1-KO, or OAS2-KO cells, demonstrating the antiviral effects of OAS3. OAS3 displayed a higher affinity for dsRNA in intact cells than either OAS1 or OAS2, consistent with its dominant role in RNase L activation. Finally, the requirement for OAS3 as the major OAS isoform responsible for RNase L activation was not restricted to A549 cells, because OAS3-KO cells derived from two other human cell lines also were deficient in RNase L activation.
Keywords: 2-5A; antiviral response; oligoadenylate synthetase 3; ribonuclease L; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

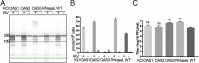



Similar articles
-
Activation of RNase L in Egyptian Rousette Bat-Derived RoNi/7 Cells Is Dependent Primarily on OAS3 and Independent of MAVS Signaling.mBio. 2019 Nov 12;10(6):e02414-19. doi: 10.1128/mBio.02414-19. mBio. 2019. PMID: 31719180 Free PMC article.
-
OAS1 and OAS3 negatively regulate the expression of chemokines and interferon-responsive genes in human macrophages.BMB Rep. 2019 Feb;52(2):133-138. doi: 10.5483/BMBRep.2019.52.2.129. BMB Rep. 2019. PMID: 30078389 Free PMC article.
-
A CRISPR-Cas9 knockout screening identifies IRF2 as a key driver of OAS3/RNase L-mediated RNA decay during viral infection.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2412725121. doi: 10.1073/pnas.2412725121. Epub 2024 Oct 30. Proc Natl Acad Sci U S A. 2024. PMID: 39475651 Free PMC article.
-
Inhibition of the OAS/RNase L pathway by viruses.Curr Opin Virol. 2015 Dec;15:19-26. doi: 10.1016/j.coviro.2015.07.002. Epub 2015 Jul 29. Curr Opin Virol. 2015. PMID: 26231767 Free PMC article. Review.
-
New insights into the role of RNase L in innate immunity.J Interferon Cytokine Res. 2011 Jan;31(1):49-57. doi: 10.1089/jir.2010.0120. Epub 2010 Dec 29. J Interferon Cytokine Res. 2011. PMID: 21190483 Free PMC article. Review.
Cited by
-
Recurrent viral capture of cellular phosphodiesterases that antagonize OAS-RNase L.Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2312691121. doi: 10.1073/pnas.2312691121. Epub 2024 Jan 26. Proc Natl Acad Sci U S A. 2024. PMID: 38277437 Free PMC article.
-
Rotavirus NSP1 Subverts the Antiviral Oligoadenylate Synthetase-RNase L Pathway by Inducing RNase L Degradation.mBio. 2022 Dec 20;13(6):e0299522. doi: 10.1128/mbio.02995-22. Epub 2022 Nov 22. mBio. 2022. PMID: 36413023 Free PMC article.
-
Impact of virus subtype and host IFNL4 genotype on large-scale RNA structure formation in the genome of hepatitis C virus.RNA. 2020 Nov;26(11):1541-1556. doi: 10.1261/rna.075465.120. Epub 2020 Aug 3. RNA. 2020. PMID: 32747607 Free PMC article.
-
Pathogen-driven CRISPR screens identify TREX1 as a regulator of DNA self-sensing during influenza virus infection.bioRxiv [Preprint]. 2023 Feb 7:2023.02.07.527556. doi: 10.1101/2023.02.07.527556. bioRxiv. 2023. Update in: Cell Host Microbe. 2023 Sep 13;31(9):1552-1567.e8. doi: 10.1016/j.chom.2023.08.001. PMID: 36798235 Free PMC article. Updated. Preprint.
-
RNase L Antiviral Activity Is Not a Critical Component of the Oas1b-Mediated Flavivirus Resistance Phenotype.J Virol. 2019 Oct 29;93(22):e00946-19. doi: 10.1128/JVI.00946-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462564 Free PMC article.
References
-
- Kristiansen H, Gad HH, Eskildsen-Larsen S, Despres P, Hartmann R. The oligoadenylate synthetase family: An ancient protein family with multiple antiviral activities. J Interferon Cytokine Res. 2011;31(1):41–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

