Molecular and Cellular Profiling of Scalp Psoriasis Reveals Differences and Similarities Compared to Skin Psoriasis
- PMID: 26849645
- PMCID: PMC4743842
- DOI: 10.1371/journal.pone.0148450
Molecular and Cellular Profiling of Scalp Psoriasis Reveals Differences and Similarities Compared to Skin Psoriasis
Abstract
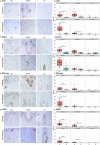
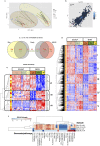
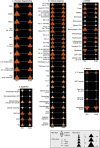
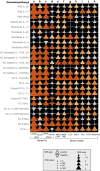
Scalp psoriasis shows a variable clinical spectrum and in many cases poses a great therapeutic challenge. However, it remains unknown whether the immune response of scalp psoriasis differs from understood pathomechanisms of psoriasis in other skin areas. We sought to determine the cellular and molecular phenotype of scalp psoriasis by performing a comparative analysis of scalp and skin using lesional and nonlesional samples from 20 Caucasian subjects with untreated moderate to severe psoriasis and significant scalp involvement and 10 control subjects without psoriasis. Our results suggest that even in the scalp, psoriasis is a disease of the inter-follicular skin. The immune mechanisms that mediate scalp psoriasis were found to be similar to those involved in skin psoriasis. However, the magnitude of dysregulation, number of differentially expressed genes, and enrichment of the psoriatic genomic fingerprint were more prominent in skin lesions. Furthermore, the scalp transcriptome showed increased modulation of several gene-sets, particularly those induced by interferon-gamma, compared with that of skin psoriasis, which was mainly associated with activation of TNFα/L-17/IL-22-induced keratinocyte response genes. We also detected differences in expression of gene-sets involving negative regulation, epigenetic regulation, epidermal differentiation, and dendritic cell or Th1/Th17/Th22-related T-cell processes.
Conflict of interest statement
Figures

Similar articles
-
Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing.J Allergy Clin Immunol. 2015 Nov;136(5):1277-87. doi: 10.1016/j.jaci.2015.06.032. Epub 2015 Aug 24. J Allergy Clin Immunol. 2015. PMID: 26316095
-
Characterization and profiling of immunomodulatory genes in resident mesenchymal stem cells reflect the Th1-Th17/Th2 imbalance of psoriasis.Arch Dermatol Res. 2014 Dec;306(10):915-20. doi: 10.1007/s00403-014-1493-3. Epub 2014 Aug 27. Arch Dermatol Res. 2014. PMID: 25160906
-
Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis.J Dermatol Sci. 2016 Mar;81(3):153-64. doi: 10.1016/j.jdermsci.2015.12.009. Epub 2015 Dec 23. J Dermatol Sci. 2016. PMID: 26794805
-
Cytokine network in psoriasis revisited.Eur Cytokine Netw. 2011 Dec;22(4):160-8. doi: 10.1684/ecn.2011.0294. Eur Cytokine Netw. 2011. PMID: 22236965 Review.
-
Psoriasis: Keratinocytes or Immune Cells - Which Is the Trigger?Dermatology. 2019;235(2):91-100. doi: 10.1159/000495291. Epub 2018 Dec 19. Dermatology. 2019. PMID: 30566935 Review.
Cited by
-
Molecular and Cellular Mechanisms of Itch in Psoriasis.Int J Mol Sci. 2020 Nov 9;21(21):8406. doi: 10.3390/ijms21218406. Int J Mol Sci. 2020. PMID: 33182442 Free PMC article. Review.
-
The Role of T Helper 22 Cells in Dermatological Disorders.Front Immunol. 2022 Jul 14;13:911546. doi: 10.3389/fimmu.2022.911546. eCollection 2022. Front Immunol. 2022. PMID: 35911703 Free PMC article. Review.
-
Bioinformatic Analysis and Translational Validation of Psoriasis Candidate Genes for Precision Medicine.Clin Cosmet Investig Dermatol. 2022 Jul 28;15:1447-1458. doi: 10.2147/CCID.S378143. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 35924255 Free PMC article.
-
Decreased A-to-I RNA editing as a source of keratinocytes' dsRNA in psoriasis.RNA. 2018 Jun;24(6):828-840. doi: 10.1261/rna.064659.117. Epub 2018 Mar 28. RNA. 2018. PMID: 29592874 Free PMC article.
-
An Overview of Contemporary and Future Therapeutic Strategies for Scalp Psoriasis.Curr Drug Targets. 2024;25(5):353-373. doi: 10.2174/0113894501292755240304063020. Curr Drug Targets. 2024. PMID: 38500274 Review.
References
-
- Farber EM, and Nall L. Natural history and treatment of scalp psoriasis. Cutis 1992. 49: 396–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

