White Matter Lipids as a Ketogenic Fuel Supply in Aging Female Brain: Implications for Alzheimer's Disease
- PMID: 26844268
- PMCID: PMC4703712
- DOI: 10.1016/j.ebiom.2015.11.002
White Matter Lipids as a Ketogenic Fuel Supply in Aging Female Brain: Implications for Alzheimer's Disease
Abstract
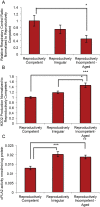
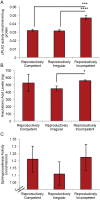
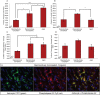
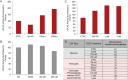
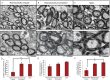
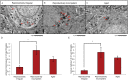
White matter degeneration is a pathological hallmark of neurodegenerative diseases including Alzheimer's. Age remains the greatest risk factor for Alzheimer's and the prevalence of age-related late onset Alzheimer's is greatest in females. We investigated mechanisms underlying white matter degeneration in an animal model consistent with the sex at greatest Alzheimer's risk. Results of these analyses demonstrated decline in mitochondrial respiration, increased mitochondrial hydrogen peroxide production and cytosolic-phospholipase-A2 sphingomyelinase pathway activation during female brain aging. Electron microscopic and lipidomic analyses confirmed myelin degeneration. An increase in fatty acids and mitochondrial fatty acid metabolism machinery was coincident with a rise in brain ketone bodies and decline in plasma ketone bodies. This mechanistic pathway and its chronologically phased activation, links mitochondrial dysfunction early in aging with later age development of white matter degeneration. The catabolism of myelin lipids to generate ketone bodies can be viewed as a systems level adaptive response to address brain fuel and energy demand. Elucidation of the initiating factors and the mechanistic pathway leading to white matter catabolism in the aging female brain provides potential therapeutic targets to prevent and treat demyelinating diseases such as Alzheimer's and multiple sclerosis. Targeting stages of disease and associated mechanisms will be critical.
Keywords: ABAD, Aβ-binding alcohol dehydrogenase; ABAD, Aβ-binding-alcohol-dehydrogenase; ACER3, alkaline ceramidase; AD, Alzheimer's disease; APO-ε4, apolipoprotein ε4; APP, amyloid precursor protein; Aging oxidative stress; Alzheimer's disease; BACE1, beta-secretase 1; BBB, blood brain barrier; CC, corpus callosum; CMRglu, cerebral glucose metabolic rate; COX, complex IV cytochrome c oxidase; CPT1, carnitine palmitoyltransferase 1; Cldn11, claudin 11; Cyp2j6, arachidonic acid epoxygenase; Cytosolic phospholipase A2; DHA, docosahexaesnoic acid; Erbb3, Erb-B2 receptor tyrosine kinase 3; FDG-PET, 2-[18F]fluoro-2-deoxy-d-glucose; GFAP, glial fibrillary acidic protein; H2O2, hydrogen peroxide; HADHA, hydroxyacyl-CoA dehydrogenase; HK, hexokinase; Ketone bodies; LC MS, liquid chromatography mass spectrometer; MAG, myelin associated glycoprotein; MBP, myelin basic protein; MCT1, monocarboxylate transporter 1; MIB, mitochondrial isolation buffer; MOG, myelin oligodendrocyte glycoprotein; MTL, medial temporal lobe; Mitochondria; NEFA, nonesterified fatty acids; Neurodegeneration; OCR, oxygen consumption rate; Olig2, oligodendrocyte transcription factor; PB, phosphate buffer; PCC, posterior cingulate; PCR, polymerase chain reaction; PDH, pyruvate dehydrogenase; PEI, polyethyleneimine; RCR, respiratory control ratio; ROS, reactive oxygen species; S1P, sphingosine; TLDA, TaqMan low density array; WM, white matter; WT, wild type; White matter; cPLA2, cytosolic phospholipase A2.
Figures
Similar articles
-
Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer's mouse brain: implication for bioenergetic intervention.PLoS One. 2013 Nov 11;8(11):e79977. doi: 10.1371/journal.pone.0079977. eCollection 2013. PLoS One. 2013. PMID: 24244584 Free PMC article.
-
Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14670-5. doi: 10.1073/pnas.0903563106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667196 Free PMC article.
-
Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies.Prog Neurobiol. 2013 Sep;108:21-43. doi: 10.1016/j.pneurobio.2013.06.004. Epub 2013 Jul 11. Prog Neurobiol. 2013. PMID: 23850509 Review.
-
Oligomeric amyloid-beta induces MAPK-mediated activation of brain cytosolic and calcium-independent phospholipase A2 in a spatial-specific manner.Acta Neuropathol Commun. 2017 Jul 27;5(1):56. doi: 10.1186/s40478-017-0460-6. Acta Neuropathol Commun. 2017. PMID: 28750656 Free PMC article.
-
A unifying hypothesis of Alzheimer's disease. IV. Causation and sequence of events.Rev Neurosci. 2000;11 Spec No:213-328. doi: 10.1515/revneuro.2000.11.s1.213. Rev Neurosci. 2000. PMID: 11065271 Review.
Cited by
-
Connections between reproductive health and cognitive aging among women enrolled in the HCHS/SOL and SOL-INCA.Alzheimers Dement. 2024 Mar;20(3):1944-1957. doi: 10.1002/alz.13575. Epub 2023 Dec 31. Alzheimers Dement. 2024. PMID: 38160447 Free PMC article.
-
Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice.J Neuroinflammation. 2021 Mar 6;18(1):66. doi: 10.1186/s12974-021-02111-4. J Neuroinflammation. 2021. PMID: 33676524 Free PMC article.
-
Human Gray and White Matter Metabolomics to Differentiate APOE and Stage Dependent Changes in Alzheimer's Disease.J Cell Immunol. 2021;3(6):397-412. doi: 10.33696/immunology.3.123. J Cell Immunol. 2021. PMID: 35265943 Free PMC article.
-
Lipid metabolism and Alzheimer's disease: clinical evidence, mechanistic link and therapeutic promise.FEBS J. 2023 Mar;290(6):1420-1453. doi: 10.1111/febs.16344. Epub 2022 Jan 18. FEBS J. 2023. PMID: 34997690 Free PMC article. Review.
-
The Role of ApoE Polymorphism in the Relationship between Serum Steroid Hormone Levels and Cognition in Older Chinese Adults: A Cross-Sectional Study.Front Endocrinol (Lausanne). 2018 Mar 6;9:71. doi: 10.3389/fendo.2018.00071. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29559956 Free PMC article.
References
-
- Alzheimer's A. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. - PubMed
-
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol. Aging. 2004;25:5–18. (author reply 49-62) - PubMed
-
- Bartzokis G., Lu P.H., Geschwind D.H., Edwards N., Mintz J., Cummings J.L. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch. Gen. Psychiatry. 2006;63:63–72. - PubMed
-
- Baumann N., Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001;81:871–927. - PubMed
-
- Beal M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58:495–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

