Hyperglycemia Increases the Production of Amyloid Beta-Peptide Leading to Decreased Endothelial Tight Junction
- PMID: 26842741
- PMCID: PMC6492831
- DOI: 10.1111/cns.12503
Hyperglycemia Increases the Production of Amyloid Beta-Peptide Leading to Decreased Endothelial Tight Junction
Abstract
Aims: Amyloid beta-peptide (Aβ), the main component of senile plaques in the Alzheimer's disease (AD) brains, is generated from sequential cleavage of amyloid precursor protein (APP) by β- and γ-secretase. Hyperglycemia in diabetes may compromise barrier integrity in endothelial cells (ECs). However, the roles of endothelial APP in response to high glucose (HG) remain to be delineated. The aims of this study were to test whether HG may increase Aβ secretion, thereby leading to heightened paracellular permeability in ECs.
Methods: We determined the effects of HG on production of Aβ, expression of full-length APP, intercellular permeability, and expression levels of specific junctional proteins in human umbilical vein endothelial cells (HUVECs).
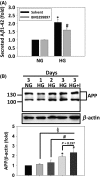
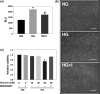
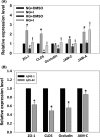
Results: HG at 30 mM significantly stimulated expression of full-length APP accompanied by heightened secretion of Aβ1-42, increased paracellular permeability, and attenuated expression of zona occluden-1 (ZO-1), claudin-5, occludin, and junctional adhesion molecule (JAM)-C in HUVECs; all of which were abolished by the γ-secretase inhibitor BMS299897. Exogenous application of Aβ1-42, but not the reverse peptide Aβ42-1, was sufficient to downregulate the expression of the same junction proteins.
Conclusion: Hyperglycemia enhances APP expression with increased Aβ production, which downregulates junctional proteins causing increased intercellular permeability in ECs.
Keywords: Alzheimer's disease; Amyloid precursor protein; High glucose; Human umbilical vein endothelial cells; Junctional permeability.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
The effect of hyperglycaemia on permeability and the expression of junctional complex molecules in human retinal and choroidal endothelial cells.Exp Eye Res. 2014 Apr;121:161-7. doi: 10.1016/j.exer.2014.02.016. Epub 2014 Mar 2. Exp Eye Res. 2014. PMID: 24594192
-
Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity.Cell Death Dis. 2017 Oct 12;8(10):e3102. doi: 10.1038/cddis.2017.491. Cell Death Dis. 2017. PMID: 29022894 Free PMC article.
-
EGb761 provides a protective effect against Aβ1-42 oligomer-induced cell damage and blood-brain barrier disruption in an in vitro bEnd.3 endothelial model.PLoS One. 2014 Nov 26;9(11):e113126. doi: 10.1371/journal.pone.0113126. eCollection 2014. PLoS One. 2014. PMID: 25426944 Free PMC article.
-
MicroRNA Regulation of Endothelial Junction Proteins and Clinical Consequence.Mediators Inflamm. 2016;2016:5078627. doi: 10.1155/2016/5078627. Epub 2016 Nov 24. Mediators Inflamm. 2016. PMID: 27999452 Free PMC article. Review.
-
The blood-brain and gut-vascular barriers: from the perspective of claudins.Tissue Barriers. 2021 Jul 3;9(3):1926190. doi: 10.1080/21688370.2021.1926190. Epub 2021 Jun 21. Tissue Barriers. 2021. PMID: 34152937 Free PMC article. Review.
Cited by
-
Melatonin: A potential nighttime guardian against Alzheimer's.Mol Psychiatry. 2025 Jan;30(1):237-250. doi: 10.1038/s41380-024-02691-6. Epub 2024 Aug 11. Mol Psychiatry. 2025. PMID: 39128995 Free PMC article. Review.
-
Role of Oxidative Stress in Tuberculosis Meningitis Infection in Diabetics.Biomedicines. 2023 Sep 19;11(9):2568. doi: 10.3390/biomedicines11092568. Biomedicines. 2023. PMID: 37761009 Free PMC article. Review.
-
Hypoxia-induced hyperpermeability of rat glomerular endothelial cells involves HIF-2α mediated changes in the expression of occludin and ZO-1.Braz J Med Biol Res. 2018;51(7):e6201. doi: 10.1590/1414-431x20186201. Epub 2018 May 17. Braz J Med Biol Res. 2018. PMID: 29791586 Free PMC article.
-
Blood-brain barrier dysfunction and recovery after ischemic stroke.Prog Neurobiol. 2018 Apr-May;163-164:144-171. doi: 10.1016/j.pneurobio.2017.10.001. Epub 2017 Oct 5. Prog Neurobiol. 2018. PMID: 28987927 Free PMC article. Review.
-
Remodeling of the Neurovascular Unit Following Cerebral Ischemia and Hemorrhage.Cells. 2022 Sep 9;11(18):2823. doi: 10.3390/cells11182823. Cells. 2022. PMID: 36139398 Free PMC article. Review.
References
-
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood–brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 2003;10:463–470. - PubMed
-
- Eringa EC, Serne EH, Meijer RI, et al. Endothelial dysfunction in (pre)diabetes: characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev Endocr Metab Disord 2013;14:39–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

