iPSC Neuronal Assay Identifies Amaryllidaceae Pharmacophore with Multiple Effects against Herpesvirus Infections
- PMID: 26819664
- PMCID: PMC4716588
- DOI: 10.1021/acsmedchemlett.5b00318
iPSC Neuronal Assay Identifies Amaryllidaceae Pharmacophore with Multiple Effects against Herpesvirus Infections
Abstract
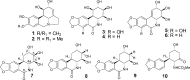
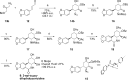
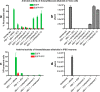
The Amaryllidaceae alkaloid trans-dihydrolycoricidine 7 and three analogues 8-10 were produced via asymmetric chemical synthesis. Alkaloid 7 proved superior to acyclovir, the current standard for herpes simplex virus, type 1 (HSV-1) infection. Compound 7 potently inhibited lytic HSV-1 infection, significantly reduced HSV-1 reactivation, and more potently inhibited varicella zoster virus (VZV) lytic infection. A configurationally defined (3R)-secondary alcohol at C3 proved crucial for efficacious inhibition of lytic HSV-1 infection.
Keywords: Amaryllidaceae; HSV-1; Herpesvirus; alkaloid; stem cells; varicella zoster.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
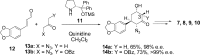

Similar articles
-
Role of the JNK Pathway in Varicella-Zoster Virus Lytic Infection and Reactivation.J Virol. 2017 Aug 10;91(17):e00640-17. doi: 10.1128/JVI.00640-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28637759 Free PMC article.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
-
[Prevention and therapy of herpesvirus infections].Zentralbl Bakteriol Mikrobiol Hyg B. 1985 Feb;180(2-3):107-20. Zentralbl Bakteriol Mikrobiol Hyg B. 1985. PMID: 2986378 Review. German.
-
Broad-spectrum non-nucleoside inhibitors of human herpesviruses.Antiviral Res. 2015 Sep;121:16-23. doi: 10.1016/j.antiviral.2015.06.005. Epub 2015 Jun 12. Antiviral Res. 2015. PMID: 26079681 Free PMC article.
-
Granzyme B Cleaves Multiple Herpes Simplex Virus 1 and Varicella-Zoster Virus (VZV) Gene Products, and VZV ORF4 Inhibits Natural Killer Cell Cytotoxicity.J Virol. 2019 Oct 29;93(22):e01140-19. doi: 10.1128/JVI.01140-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462576 Free PMC article.
Cited by
-
R430: A potent inhibitor of DNA and RNA viruses.Sci Rep. 2018 Nov 9;8(1):16662. doi: 10.1038/s41598-018-33904-y. Sci Rep. 2018. PMID: 30413769 Free PMC article.
-
Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived from Induced Pluripotent Stem Cells.J Virol. 2019 Apr 17;93(9):e00111-19. doi: 10.1128/JVI.00111-19. Print 2019 May 1. J Virol. 2019. PMID: 30787148 Free PMC article.
-
Using 2D and 3D pluripotent stem cell models to study neurotropic viruses.Front Virol. 2022;2:869657. doi: 10.3389/fviro.2022.869657. Epub 2022 Jul 29. Front Virol. 2022. PMID: 36325520 Free PMC article.
-
Stereoselective synthesis of trans-dihydronarciclasine derivatives containing a 1,4-benzodioxane moiety.Monatsh Chem. 2018;149(12):2265-2285. doi: 10.1007/s00706-018-2287-7. Epub 2018 Oct 26. Monatsh Chem. 2018. PMID: 32214482 Free PMC article.
-
Medicinal plants and natural compounds against acyclovir-resistant HSV infections.Front Microbiol. 2022 Oct 10;13:1025605. doi: 10.3389/fmicb.2022.1025605. eCollection 2022. Front Microbiol. 2022. PMID: 36299732 Free PMC article. Review.
References
-
- Evidente A.; Kireev A. S.; Jenkins A. R.; Romero A. E.; Steelant W. F. A.; van Slambrouck S.; Kornienko A. Biological evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives: discovery of novel leads for anticancer drug design. Planta Med. 2009, 75, 501–7. 10.1055/s-0029-1185340. - DOI - PMC - PubMed
-
- Gabrielsen B.; Monath T. P.; Huggins J. W.; Kefauver D. F.; Pettit G. R.; Groszek G.; Hollingshead M.; Kirsi J. J.; Shannon W. M.; Schubert E. M.; DaRe J.; Ugarkar B.; Ussery M.; Phelan M. J. Antiviral (RNA) activity of selected Amaryllidaceae isoquinoline constituents and synthesis of related substances. J. Nat. Prod. 1992, 55, 1569–81. 10.1021/np50089a003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

