Suppression of Type I Interferon Production by Human T-Cell Leukemia Virus Type 1 Oncoprotein Tax through Inhibition of IRF3 Phosphorylation
- PMID: 26819312
- PMCID: PMC4810532
- DOI: 10.1128/JVI.00129-16
Suppression of Type I Interferon Production by Human T-Cell Leukemia Virus Type 1 Oncoprotein Tax through Inhibition of IRF3 Phosphorylation
Abstract
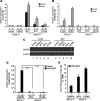
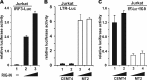
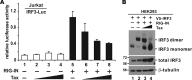
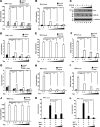
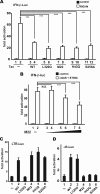
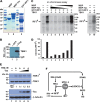
Infection with human T-cell leukemia virus type 1 (HTLV-1) is associated with adult T-cell leukemia (ATL) and tropical spastic paraparesis. Type I interferons (IFNs) are key effectors of the innate antiviral response, and IFN-α combined with the nucleoside reverse transcriptase inhibitor zidovudine is considered the standard first-line therapy for ATL. HTLV-1 oncoprotein Tax is known to suppress innate IFN production and response but the underlying mechanisms remain to be fully established. In this study, we report on the suppression of type I IFN production by HTLV-1 Tax through interaction with and inhibition of TBK1 kinase that phosphorylates IRF3. Induced transcription of IFN-β was severely impaired in HTLV-1-transformed ATL cells and freshly infected T lymphocytes. The ability to suppress IRF3 activation was ascribed to Tax. The expression of Tax alone sufficiently repressed the induction of IFN production by RIG-I plus PACT, cGAMP synthase plus STING, TBK1, IKKε, IRF3, and IRF7, but not by IRF3-5D, a dominant-active phosphomimetic mutant. This suggests that Tax perturbs IFN production at the step of IRF3 phosphorylation. Tax mutants deficient for CREB or NF-κB activation were fully competent in the suppression of IFN production. Coimmunoprecipitation experiments confirmed the association of Tax with TBK1, IKKε, STING, and IRF3.In vitrokinase assay indicated an inhibitory effect of Tax on TBK1-mediated phosphorylation of IRF3. Taken together, our findings suggested a new mechanism by which HTLV-1 oncoprotein Tax circumvents the production of type I IFNs in infected cells. Our findings have implications in therapeutic intervention of ATL.
Importance: Human T-cell leukemia virus type 1 (HTLV-1) is the cause of adult T-cell leukemia (ATL), an aggressive and fatal blood cancer, as well as another chronic disabling disease of the spinal cord. Treatments are unsatisfactory, and options are limited. A combination of antiviral cellular protein alpha interferon and zidovudine, which is an inhibitor of a viral enzyme called reverse transcriptase, has been recommended as the standard first-line therapy for ATL. Exactly how HTLV-1 interacts with the cellular machinery for interferon production and action is not well understood. Our work sheds light on the mechanism of action for the inhibition of interferon production by an HTLV-1 oncogenic protein called Tax. Our findings might help to improve interferon-based anti-HTLV-1 and anti-ATL therapy.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Positive and Negative Regulation of Type I Interferons by the Human T Cell Leukemia Virus Antisense Protein HBZ.J Virol. 2017 Sep 27;91(20):e00853-17. doi: 10.1128/JVI.00853-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768861 Free PMC article.
-
Human T-cell leukemia virus type 1 Tax oncoprotein represses the expression of the BCL11B tumor suppressor in T-cells.Cancer Sci. 2015 Apr;106(4):461-5. doi: 10.1111/cas.12618. Epub 2015 Mar 16. Cancer Sci. 2015. PMID: 25613934 Free PMC article.
-
Identification of TBK1 and IKKε, the non-canonical IκB kinases, as crucial pro-survival factors in HTLV-1-transformed T lymphocytes.Leuk Res. 2016 Jul;46:37-44. doi: 10.1016/j.leukres.2016.04.012. Epub 2016 Apr 16. Leuk Res. 2016. PMID: 27123832 Free PMC article.
-
HTLV-1 Tax and adult T-cell leukemia.Front Biosci. 2007 Jan 1;12:1496-507. doi: 10.2741/2163. Front Biosci. 2007. PMID: 17127397 Review.
-
The multifaceted oncoprotein Tax: subcellular localization, posttranslational modifications, and NF-κB activation.Adv Cancer Res. 2012;113:85-120. doi: 10.1016/B978-0-12-394280-7.00003-8. Adv Cancer Res. 2012. PMID: 22429853 Review.
Cited by
-
Arenaviral Nucleoproteins Suppress PACT-Induced Augmentation of RIG-I Function To Inhibit Type I Interferon Production.J Virol. 2018 Jun 13;92(13):e00482-18. doi: 10.1128/JVI.00482-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29669840 Free PMC article.
-
TANK-Binding Kinase 1 (TBK1) Isoforms Negatively Regulate Type I Interferon Induction by Inhibiting TBK1-IRF3 Interaction and IRF3 Phosphorylation.Front Immunol. 2018 Jan 30;9:84. doi: 10.3389/fimmu.2018.00084. eCollection 2018. Front Immunol. 2018. PMID: 29441066 Free PMC article.
-
Impact of host immunity on HTLV-1 pathogenesis: potential of Tax-targeted immunotherapy against ATL.Retrovirology. 2019 Aug 22;16(1):23. doi: 10.1186/s12977-019-0484-z. Retrovirology. 2019. PMID: 31438973 Free PMC article. Review.
-
Pentosan Polysulfate Demonstrates Anti-human T-Cell Leukemia Virus Type 1 Activities In Vitro and In Vivo.J Virol. 2019 Jul 30;93(16):e00413-19. doi: 10.1128/JVI.00413-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31167921 Free PMC article.
-
Interplay between innate immunity and the viral oncoproteins Tax and HBZ in the pathogenesis and therapeutic response of HTLV-1 associated adult T cell leukemia.Front Immunol. 2022 Jul 22;13:957535. doi: 10.3389/fimmu.2022.957535. eCollection 2022. Front Immunol. 2022. PMID: 35935975 Free PMC article. Review.
References
-
- Gill PS, Harrington W Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, Bernstein-Singer M, Espina BM, Cabral L, Allen S, Kornblau S, Pike MC, Levine AM. 1995. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon α and zidovudine. N Engl J Med 332:1744–1748. doi:10.1056/NEJM199506293322603. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

