Conformational Changes in Transmembrane Domain 4 of Presenilin 1 Are Associated with Altered Amyloid-β 42 Production
- PMID: 26818522
- PMCID: PMC6604815
- DOI: 10.1523/JNEUROSCI.5090-14.2016
Conformational Changes in Transmembrane Domain 4 of Presenilin 1 Are Associated with Altered Amyloid-β 42 Production
Abstract
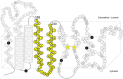
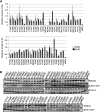
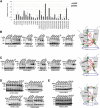
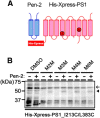
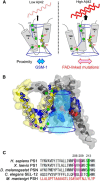
γ-Secretase is an intramembrane-cleaving protease that produces amyloid-β peptide 42 (Aβ42), which is the toxic and aggregation-prone species of Aβ that causes Alzheimer's disease. Here, we used the substituted cysteine accessibility method to analyze the structure of transmembrane domains (TMDs) 4 and 5 of human presenilin 1 (PS1), a catalytic subunit of γ-secretase. We revealed that TMD4 and TMD5 face the intramembranous hydrophilic milieu together with TMD1, TMD6, TMD7, and TMD9 of PS1 to form the catalytic pore structure. Notably, we found a correlation in the distance between the cytosolic sides of TMD4/TMD7 and Aβ42 production levels, suggesting that allosteric conformational changes of the cytosolic side of TMD4 affect Aβ42-generating γ-secretase activity. Our results provide new insights into the relationship between the structure and activity of human PS1.
Significance statement: Modulation of γ-secretase activity to reduce toxic amyloid-β peptide species is one plausible therapeutic approaches for Alzheimer's disease. However, precise mechanistic information of γ-secretase still remains unclear. Here we identified the conformational changes in transmembrane domains of presenilin 1 that affect the proteolytic activity of the γ-secretase. Our results highlight the importance of understanding the structural dynamics of presenilin 1 in drug development against Alzheimer's disease.
Keywords: allosteric change; amyloid; enzyme; membrane protein; protease; secretase.
Copyright © 2016 the authors 0270-6474/16/361362-11$15.00/0.
Figures



Similar articles
-
Conformational Dynamics of Transmembrane Domain 3 of Presenilin 1 Is Associated with the Trimming Activity of γ-Secretase.J Neurosci. 2019 Oct 23;39(43):8600-8610. doi: 10.1523/JNEUROSCI.0838-19.2019. Epub 2019 Sep 16. J Neurosci. 2019. PMID: 31527118 Free PMC article.
-
Activation of γ-Secretase Trimming Activity by Topological Changes of Transmembrane Domain 1 of Presenilin 1.J Neurosci. 2017 Dec 13;37(50):12272-12280. doi: 10.1523/JNEUROSCI.1628-17.2017. Epub 2017 Nov 8. J Neurosci. 2017. PMID: 29118109 Free PMC article.
-
Participation of transmembrane domain 1 of presenilin 1 in the catalytic pore structure of the γ-secretase.J Neurosci. 2010 Nov 24;30(47):15943-50. doi: 10.1523/JNEUROSCI.3318-10.2010. J Neurosci. 2010. PMID: 21106832 Free PMC article.
-
Dynamic Nature of presenilin1/γ-Secretase: Implication for Alzheimer's Disease Pathogenesis.Mol Neurobiol. 2018 Mar;55(3):2275-2284. doi: 10.1007/s12035-017-0487-5. Epub 2017 Mar 22. Mol Neurobiol. 2018. PMID: 28332150 Free PMC article. Review.
-
Toward the structure of presenilin/γ-secretase and presenilin homologs.Biochim Biophys Acta. 2013 Dec;1828(12):2886-97. doi: 10.1016/j.bbamem.2013.04.015. Biochim Biophys Acta. 2013. PMID: 24099007 Free PMC article. Review.
Cited by
-
Interaction of Substrates with γ-Secretase at the Level of Individual Transmembrane Helices-A Methodological Approach.Int J Mol Sci. 2023 Sep 21;24(18):14396. doi: 10.3390/ijms241814396. Int J Mol Sci. 2023. PMID: 37762696 Free PMC article.
-
An internal docking site stabilizes substrate binding to γ-secretase: Analysis by molecular dynamics simulations.Biophys J. 2022 Jun 21;121(12):2330-2344. doi: 10.1016/j.bpj.2022.05.023. Epub 2022 May 20. Biophys J. 2022. PMID: 35598043 Free PMC article.
-
Characterizing the structural ensemble of γ-secretase using a multiscale molecular dynamics approach.Chem Sci. 2017 Aug 1;8(8):5576-5584. doi: 10.1039/c7sc00980a. Epub 2017 Jun 5. Chem Sci. 2017. PMID: 28970936 Free PMC article.
-
Different transmembrane domains determine the specificity and efficiency of the cleavage activity of the γ-secretase subunit presenilin.J Biol Chem. 2023 May;299(5):104626. doi: 10.1016/j.jbc.2023.104626. Epub 2023 Mar 20. J Biol Chem. 2023. PMID: 36944398 Free PMC article.
-
Genetics, Functions, and Clinical Impact of Presenilin-1 (PSEN1) Gene.Int J Mol Sci. 2022 Sep 19;23(18):10970. doi: 10.3390/ijms231810970. Int J Mol Sci. 2022. PMID: 36142879 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
