Decrease of myofiber branching via muscle-specific expression of the olfactory receptor mOR23 in dystrophic muscle leads to protection against mechanical stress
- PMID: 26798450
- PMCID: PMC4721043
- DOI: 10.1186/s13395-016-0077-7
Decrease of myofiber branching via muscle-specific expression of the olfactory receptor mOR23 in dystrophic muscle leads to protection against mechanical stress
Abstract
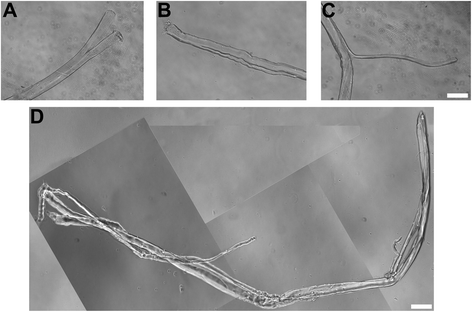
Background: Abnormal branched myofibers within skeletal muscles are commonly found in diverse animal models of muscular dystrophy as well as in patients. Branched myofibers from dystrophic mice are more susceptible to break than unbranched myofibers suggesting that muscles containing a high percentage of these myofibers are more prone to injury. Previous studies showed ubiquitous over-expression of mouse olfactory receptor 23 (mOR23), a G protein-coupled receptor, in wild type mice decreased myofiber branching. Whether mOR23 over-expression specifically in skeletal muscle cells is sufficient to mitigate myofiber branching in dystrophic muscle is unknown.
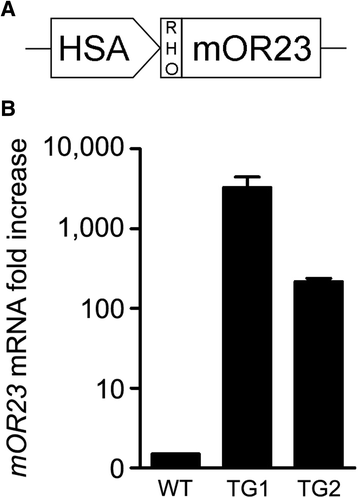
Methods: We created a novel transgenic mouse over-expressing mOR23 specifically in muscle cells and then bred with dystrophic (mdx) mice. Myofiber branching was analyzed in these two transgenic mice and membrane integrity was assessed by Evans blue dye fluorescence.
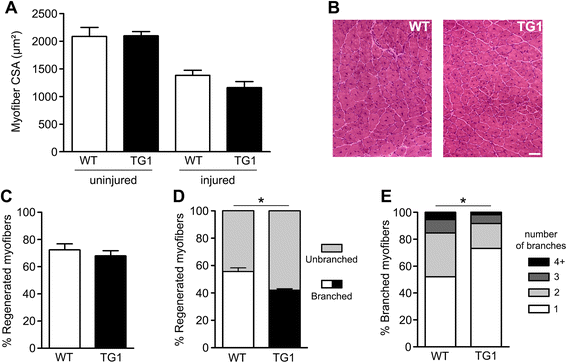
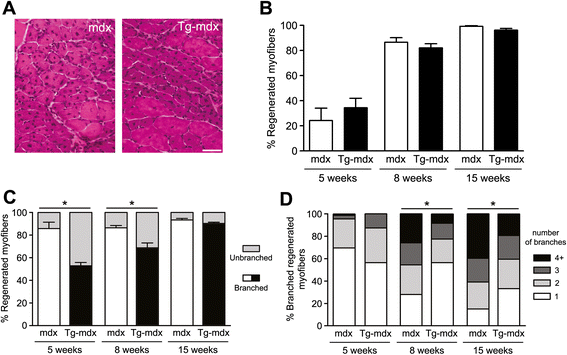
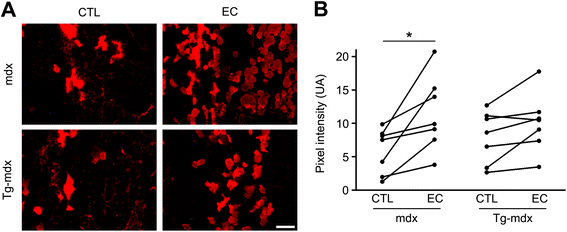
Results: mOR23 over-expression in muscle led to a decrease of myofiber branching after muscle regeneration in non-dystrophic mouse muscles and reduced the severity of myofiber branching in mdx mouse muscles. Muscles from mdx mouse over-expressing mOR23 significantly exhibited less damage to eccentric contractions than control mdx muscles.
Conclusions: The decrease of myofiber branching in mdx mouse muscles over-expressing mOR23 reduced the amount of membrane damage induced by mechanical stress. These results suggest that modifying myofiber branching in dystrophic patients, while not preventing degeneration, could be beneficial for mitigating some of the effects of the disease process.
Keywords: Mechanical stress; Muscle regeneration; Muscular dystrophy; Myofiber branching; mOR23.
Figures
Similar articles
-
Alterations in Notch signalling in skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne muscular dystrophy.Exp Physiol. 2014 Apr;99(4):675-87. doi: 10.1113/expphysiol.2013.077255. Epub 2014 Jan 17. Exp Physiol. 2014. PMID: 24443351
-
Incidence and severity of myofiber branching with regeneration and aging.Skelet Muscle. 2014 May 15;4:9. doi: 10.1186/2044-5040-4-9. eCollection 2014. Skelet Muscle. 2014. PMID: 24855558 Free PMC article.
-
Forced myofiber regeneration promotes dystrophin gene transfer and improved muscle function despite advanced disease in old dystrophic mice.Mol Ther. 2001 Nov;4(5):499-507. doi: 10.1006/mthe.2001.0482. Mol Ther. 2001. PMID: 11708887
-
Functional characteristics of dystrophic skeletal muscle: insights from animal models.J Appl Physiol (1985). 2002 Aug;93(2):407-17. doi: 10.1152/japplphysiol.01242.2001. J Appl Physiol (1985). 2002. PMID: 12133845 Review.
-
The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy.Exp Physiol. 2011 Jun;96(6):564-71. doi: 10.1113/expphysiol.2010.056713. Epub 2011 Mar 18. Exp Physiol. 2011. PMID: 21421700 Review.
Cited by
-
Sarcomeric deficits underlie MYBPC1-associated myopathy with myogenic tremor.JCI Insight. 2021 Oct 8;6(19):e147612. doi: 10.1172/jci.insight.147612. JCI Insight. 2021. PMID: 34437302 Free PMC article.
-
Angiotensin II Type 1 Receptor-associated Protein Inhibits Angiotensin II-induced Insulin Resistance with Suppression of Oxidative Stress in Skeletal Muscle Tissue.Sci Rep. 2018 Feb 12;8(1):2846. doi: 10.1038/s41598-018-21270-8. Sci Rep. 2018. PMID: 29434287 Free PMC article.
-
Muscle Fiber Splitting Is a Physiological Response to Extreme Loading in Animals.Exerc Sport Sci Rev. 2019 Apr;47(2):108-115. doi: 10.1249/JES.0000000000000181. Exerc Sport Sci Rev. 2019. PMID: 30640746 Free PMC article. Review.
-
Olfactory, Taste, and Photo Sensory Receptors in Non-sensory Organs: It Just Makes Sense.Front Physiol. 2018 Nov 27;9:1673. doi: 10.3389/fphys.2018.01673. eCollection 2018. Front Physiol. 2018. PMID: 30542293 Free PMC article. Review.
-
Lifetime analysis of mdx skeletal muscle reveals a progressive pathology that leads to myofiber loss.Sci Rep. 2020 Oct 14;10(1):17248. doi: 10.1038/s41598-020-74192-9. Sci Rep. 2020. PMID: 33057110 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

