Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis
- PMID: 26797213
- PMCID: PMC4787611
- DOI: 10.1093/cid/ciw027
Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis
Abstract
Background: Maternal immunization against pertussis is currently recommended after the 26th gestational week (GW). Data on the optimal timing of maternal immunization are inconsistent.
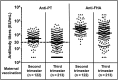
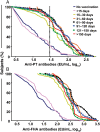
Methods: We conducted a prospective observational noninferiority study comparing the influence of second-trimester (GW 13-25) vs third-trimester (≥GW 26) tetanus-diphtheria-acellular pertussis (Tdap) immunization in pregnant women who delivered at term. Geometric mean concentrations (GMCs) of cord blood antibodies to recombinant pertussis toxin (PT) and filamentous hemagglutinin (FHA) were assessed by enzyme-linked immunosorbent assay. The primary endpoint were GMCs and expected infant seropositivity rates, defined by birth anti-PT >30 enzyme-linked immunosorbent assay units (EU)/mL to confer seropositivity until 3 months of age.
Results: We included 335 women (mean age, 31.0 ± 5.1 years; mean gestational age, 39.3 ± 1.3 GW) previously immunized with Tdap in the second (n = 122) or third (n = 213) trimester. Anti-PT and anti-FHA GMCs were higher following second- vs third-trimester immunization (PT: 57.1 EU/mL [95% confidence interval {CI}, 47.8-68.2] vs 31.1 EU/mL [95% CI, 25.7-37.7], P < .001; FHA: 284.4 EU/mL [95% CI, 241.3-335.2] vs 140.2 EU/mL [95% CI, 115.3-170.3], P < .001). The adjusted GMC ratios after second- vs third-trimester immunization differed significantly (PT: 1.9 [95% CI, 1.4-2.5]; FHA: 2.2 [95% CI, 1.7-3.0], P < .001). Expected infant seropositivity rates reached 80% vs 55% following second- vs third-trimester immunization (adjusted odds ratio, 3.7 [95% CI, 2.1-6.5], P < .001).
Conclusions: Early second-trimester maternal Tdap immunization significantly increased neonatal antibodies. Recommending immunization from the second trimester onward would widen the immunization opportunity window and could improve seroprotection.
Keywords: maternal antibodies; maternal immunization; neonates; pertussis; pregnancy.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Optimal Timing of Immunization Against Pertussis During Pregnancy.Clin Infect Dis. 2016 Jul 1;63(1):143-4. doi: 10.1093/cid/ciw233. Epub 2016 Apr 18. Clin Infect Dis. 2016. PMID: 27090990 No abstract available.
-
Reply to Abu Raya et al.Clin Infect Dis. 2016 Jul 1;63(1):144-5. doi: 10.1093/cid/ciw235. Epub 2016 Apr 18. Clin Infect Dis. 2016. PMID: 27090994 No abstract available.
Similar articles
-
Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants.Clin Infect Dis. 2013 Feb;56(4):539-44. doi: 10.1093/cid/cis923. Epub 2012 Oct 24. Clin Infect Dis. 2013. PMID: 23097585
-
Association Between Third-Trimester Tdap Immunization and Neonatal Pertussis Antibody Concentration.JAMA. 2018 Oct 9;320(14):1464-1470. doi: 10.1001/jama.2018.14298. JAMA. 2018. PMID: 30304426 Free PMC article.
-
The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels - a prospective study.Vaccine. 2014 Oct 7;32(44):5787-93. doi: 10.1016/j.vaccine.2014.08.038. Epub 2014 Aug 28. Vaccine. 2014. PMID: 25173476
-
Neonatal pertussis, cocooning and maternal immunization.Expert Rev Vaccines. 2014 Sep;13(9):1107-14. doi: 10.1586/14760584.2014.944509. Epub 2014 Jul 30. Expert Rev Vaccines. 2014. PMID: 25075629 Review.
-
Review of vaccination in pregnancy to prevent pertussis in early infancy.J Med Microbiol. 2018 Oct;67(10):1426-1456. doi: 10.1099/jmm.0.000829. Epub 2018 Sep 17. J Med Microbiol. 2018. PMID: 30222536 Review.
Cited by
-
Safety and immunogenicity of a single dose of Tdap compared to Td in pregnant women in Mali and 3 its effect on infant immune responses: a single-centre, randomised, double-blind, active-controlled phase 2 study.EClinicalMedicine. 2024 Mar 28;71:102556. doi: 10.1016/j.eclinm.2024.102556. eCollection 2024 May. EClinicalMedicine. 2024. PMID: 38586589 Free PMC article.
-
Are pertussis cases reported too late for public health interventions? Retrospective analysis of cases in London and South East England, 2010 to 2015.Euro Surveill. 2017 Jul 20;22(29):30577. doi: 10.2807/1560-7917.ES.2017.22.29.30577. Euro Surveill. 2017. PMID: 28749334 Free PMC article.
-
Pertussis seroepidemiology in women and their infants in Sarlahi District, Nepal.Vaccine. 2017 Dec 4;35(48 Pt B):6766-6773. doi: 10.1016/j.vaccine.2017.09.074. Epub 2017 Oct 14. Vaccine. 2017. PMID: 29037576 Free PMC article. Clinical Trial.
-
Understanding Early-Life Adaptive Immunity to Guide Interventions for Pediatric Health.Front Immunol. 2021 Jan 21;11:595297. doi: 10.3389/fimmu.2020.595297. eCollection 2020. Front Immunol. 2021. PMID: 33552052 Free PMC article. Review.
-
Experience and challenges on influenza and pertussis vaccination in pregnant women.Hum Vaccin Immunother. 2018;14(9):2183-2188. doi: 10.1080/21645515.2018.1483810. Epub 2018 Jul 24. Hum Vaccin Immunother. 2018. PMID: 30024822 Free PMC article. Review.
References
-
- Jakinovich A, Sood SK. Pertussis: still a cause of death, seven decades into vaccination. Curr Opin Pediatr 2014; 26:597–604. - PubMed
-
- Winter K, Harriman K, Zipprich J et al. . California pertussis epidemic, 2010. J Pediatr 2012; 161:1091–6. - PubMed
-
- Health Protection Agency. Confirmed pertussis in England and Wales: data to end-December 2012. Health Protection Report, 2013; 7.
-
- Centers for Disease Control and Prevention. Pertussis outbreak trends. Available at: http://www.cdc.gov/pertussis/outbreaks/trends.html Accessed 1 November 2015.
-
- Tiwari TS, Baughman AL, Clark TA. First pertussis vaccine dose and prevention of infant mortality. Pediatrics 2015; 135:990–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

