STIM and Orai1 Variants in Store-Operated Calcium Entry
- PMID: 26793113
- PMCID: PMC4710697
- DOI: 10.3389/fphar.2015.00325
STIM and Orai1 Variants in Store-Operated Calcium Entry
Abstract
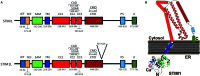
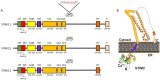
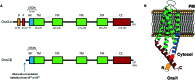
Store-operated Ca(2+) entry (SOCE) is an ubiquitous mechanism for Ca(2+) entry in eukaryotic cells. This route for Ca(2+) influx is regulated by the filling state of the intracellular Ca(2+) stores communicated to the plasma membrane channels by the proteins of the Stromal Interaction Molecule (STIM) family, STIM1, and STIM2. Store-dependent, STIM1-modulated, channels include the Ca(2+) release-activated Ca(2+) channels, comprised of subunits of Orai proteins, as well as the store-operated Ca(2+) (SOC) channels, involving Orai1, and members of the canonical transient receptor potential family of proteins. Recent studies have revealed the expression of splice variants of STIM1, STIM2, and Orai1 in different cell types. While certain variants are ubiquitously expressed, others, such as STIM1L, show a more restricted expression. The splice variants for STIM and Orai1 proteins exhibit significant functional differences and reveal that alternative splicing enhance the functional diversity of STIM1, STIM2, and Orai1 genes to modulate the dynamics of Ca(2+) signals.
Keywords: STIM1; STIM2; calcium entry; orai1; splice variants.
Figures
Similar articles
-
Store-Independent Orai Channels Regulated by STIM.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
-
Studies of Structure-Function and Subunit Composition of Orai/STIM Channel.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. PMID: 30299645 Free Books & Documents. Review.
-
Pharmacology of Store-Operated Calcium Entry Channels.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 16. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 16. PMID: 30299647 Free Books & Documents. Review.
-
Regulation and Role of Store-Operated Ca2+ Entry in Cellular Proliferation.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 12. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 12. PMID: 30299656 Free Books & Documents. Review.
-
STIM1, STIM2, and Orai1 regulate store-operated calcium entry and purinergic activation of microglia.Glia. 2015 Apr;63(4):652-63. doi: 10.1002/glia.22775. Epub 2014 Dec 3. Glia. 2015. PMID: 25471906
Cited by
-
The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells.Front Physiol. 2018 Mar 21;9:257. doi: 10.3389/fphys.2018.00257. eCollection 2018. Front Physiol. 2018. PMID: 29618985 Free PMC article. Review.
-
Regulation of store-operated Ca2+ entry by IP3 receptors independent of their ability to release Ca2.Elife. 2023 Jul 19;12:e80447. doi: 10.7554/eLife.80447. Elife. 2023. PMID: 37466241 Free PMC article.
-
Structural basis of TRPC4 regulation by calmodulin and pharmacological agents.Elife. 2020 Nov 25;9:e60603. doi: 10.7554/eLife.60603. Elife. 2020. PMID: 33236980 Free PMC article.
-
Calcium Signaling Is Dispensable for Receptor Regulation of Endothelial Barrier Function.J Biol Chem. 2016 Oct 28;291(44):22894-22912. doi: 10.1074/jbc.M116.756114. Epub 2016 Sep 13. J Biol Chem. 2016. PMID: 27624938 Free PMC article.
-
CRAC and SK Channels: Their Molecular Mechanisms Associated with Cancer Cell Development.Cancers (Basel). 2022 Dec 23;15(1):101. doi: 10.3390/cancers15010101. Cancers (Basel). 2022. PMID: 36612099 Free PMC article. Review.
References
-
- Baba Y., Hayashi K., Fujii Y., Mizushima A., Watarai H., Wakamori M., et al. (2006). Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 103 16704–16709. 10.1073/pnas.0608358103 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

