Cryo-electron microscopy structure of the TRPV2 ion channel
- PMID: 26779611
- PMCID: PMC4876856
- DOI: 10.1038/nsmb.3159
Cryo-electron microscopy structure of the TRPV2 ion channel
Abstract
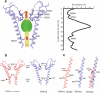
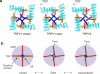
Transient receptor potential vanilloid (TRPV) cation channels are polymodal sensors involved in a variety of physiological processes. TRPV2, a member of the TRPV family, is regulated by temperature, by ligands, such as probenecid and cannabinoids, and by lipids. TRPV2 has been implicated in many biological functions, including somatosensation, osmosensation and innate immunity. Here we present the atomic model of rabbit TRPV2 in its putative desensitized state, as determined by cryo-EM at a nominal resolution of ∼4 Å. In the TRPV2 structure, the transmembrane segment 6 (S6), which is involved in gate opening, adopts a conformation different from the one observed in TRPV1. Structural comparisons of TRPV1 and TRPV2 indicate that a rotation of the ankyrin-repeat domain is coupled to pore opening via the TRP domain, and this pore opening can be modulated by rearrangements in the secondary structure of S6.
Figures




Similar articles
-
Structure of the full-length TRPV2 channel by cryo-EM.Nat Commun. 2016 Mar 29;7:11130. doi: 10.1038/ncomms11130. Nat Commun. 2016. PMID: 27021073 Free PMC article.
-
Symmetry transitions during gating of the TRPV2 ion channel in lipid membranes.Elife. 2019 May 15;8:e45779. doi: 10.7554/eLife.45779. Elife. 2019. PMID: 31090543 Free PMC article.
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
Structural insight into the assembly of TRPV channels.Structure. 2014 Feb 4;22(2):260-8. doi: 10.1016/j.str.2013.11.008. Epub 2013 Dec 26. Structure. 2014. PMID: 24373766 Free PMC article.
-
High-resolution structures of transient receptor potential vanilloid channels: Unveiling a functionally diverse group of ion channels.Protein Sci. 2020 Jul;29(7):1569-1580. doi: 10.1002/pro.3861. Epub 2020 Apr 11. Protein Sci. 2020. PMID: 32232875 Free PMC article. Review.
Cited by
-
The Structure of the Polycystic Kidney Disease Channel PKD2 in Lipid Nanodiscs.Cell. 2016 Oct 20;167(3):763-773.e11. doi: 10.1016/j.cell.2016.09.048. Cell. 2016. PMID: 27768895 Free PMC article.
-
Structural Variability in the RLR-MAVS Pathway and Sensitive Detection of Viral RNAs.Med Chem. 2019;15(5):443-458. doi: 10.2174/1573406415666181219101613. Med Chem. 2019. PMID: 30569868 Free PMC article. Review.
-
Involvement of calcium channels in the regulation of adipogenesis.Adipocyte. 2020 Dec;9(1):132-141. doi: 10.1080/21623945.2020.1738792. Adipocyte. 2020. PMID: 32175809 Free PMC article. Review.
-
Transient Receptor Potential Channels and Calcium Signaling.Cold Spring Harb Perspect Biol. 2019 Jun 3;11(6):a035048. doi: 10.1101/cshperspect.a035048. Cold Spring Harb Perspect Biol. 2019. PMID: 30910771 Free PMC article. Review.
-
Structural and Evolutionary Insights Point to Allosteric Regulation of TRP Ion Channels.Acc Chem Res. 2019 Jun 18;52(6):1643-1652. doi: 10.1021/acs.accounts.9b00075. Epub 2019 May 31. Acc Chem Res. 2019. PMID: 31149807 Free PMC article.
References
-
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–647. - PubMed
-
- Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

