Immunogenicity and efficacy of alphavirus-derived replicon vaccines for respiratory syncytial virus and human metapneumovirus in nonhuman primates
- PMID: 26772634
- PMCID: PMC4731299
- DOI: 10.1016/j.vaccine.2015.12.045
Immunogenicity and efficacy of alphavirus-derived replicon vaccines for respiratory syncytial virus and human metapneumovirus in nonhuman primates
Abstract
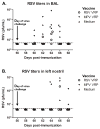
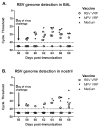
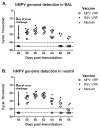
Human respiratory syncytial virus (hRSV) and human metapneumovirus (hMPV) are major causes of illness among children, the elderly, and the immunocompromised. No vaccine has been licensed for protection against either of these viruses. We tested the ability of two Venezuelan equine encephalitis virus-based viral replicon particle (VEE-VRP) vaccines that express the hRSV or hMPV fusion (F) protein to confer protection against hRSV or hMPV in African green monkeys. Animals immunized with VEE-VRP vaccines developed RSV or MPV F-specific antibodies and serum neutralizing activity. Compared to control animals, immunized animals were better able to control viral load in the respiratory mucosa following challenge and had lower levels of viral genome in nasopharyngeal and bronchoalveolar lavage fluids. The high level of immunogenicity and protective efficacy induced by these vaccine candidates in nonhuman primates suggest that they hold promise for further development.
Keywords: Metapneumovirus; Nonhuman primates; Replicon particles; Respiratory syncytial virus; Vaccine.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
An alphavirus replicon-based human metapneumovirus vaccine is immunogenic and protective in mice and cotton rats.J Virol. 2008 Nov;82(22):11410-8. doi: 10.1128/JVI.01688-08. Epub 2008 Sep 10. J Virol. 2008. PMID: 18786987 Free PMC article.
-
Cross-neutralization of four paramyxoviruses by a human monoclonal antibody.Nature. 2013 Sep 19;501(7467):439-43. doi: 10.1038/nature12442. Epub 2013 Aug 18. Nature. 2013. PMID: 23955151
-
Alphavirus replicon particles encoding the fusion or attachment glycoproteins of respiratory syncytial virus elicit protective immune responses in BALB/c mice and functional serum antibodies in rhesus macaques.Vaccine. 2007 Oct 10;25(41):7132-44. doi: 10.1016/j.vaccine.2007.07.065. Epub 2007 Aug 21. Vaccine. 2007. PMID: 17850933
-
Induction of Trained Immunity by Recombinant Vaccines.Front Immunol. 2021 Jan 7;11:611946. doi: 10.3389/fimmu.2020.611946. eCollection 2020. Front Immunol. 2021. PMID: 33584692 Free PMC article. Review.
-
Structural basis for respiratory syncytial virus and human metapneumovirus neutralization.Curr Opin Virol. 2023 Aug;61:101337. doi: 10.1016/j.coviro.2023.101337. Curr Opin Virol. 2023. PMID: 37544710 Free PMC article. Review.
Cited by
-
Non-human primate models of human respiratory infections.Mol Immunol. 2021 Jul;135:147-164. doi: 10.1016/j.molimm.2021.04.010. Epub 2021 Apr 23. Mol Immunol. 2021. PMID: 33895579 Free PMC article. Review.
-
Prophylactic and therapeutic approaches for human metapneumovirus.Virusdisease. 2018 Dec;29(4):434-444. doi: 10.1007/s13337-018-0498-5. Epub 2018 Oct 20. Virusdisease. 2018. PMID: 30539045 Free PMC article. Review.
-
Replicon RNA Viral Vectors as Vaccines.Vaccines (Basel). 2016 Nov 7;4(4):39. doi: 10.3390/vaccines4040039. Vaccines (Basel). 2016. PMID: 27827980 Free PMC article. Review.
-
Peripheral Blood Biomarkers of Disease Outcome in a Monkey Model of Rift Valley Fever Encephalitis.J Virol. 2018 Jan 17;92(3):e01662-17. doi: 10.1128/JVI.01662-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29118127 Free PMC article.
-
Potential use of noncoding RNAs and innovative therapeutic strategies to target the 5'UTR of SARS-CoV-2.Epigenomics. 2020 Aug;12(15):1349-1361. doi: 10.2217/epi-2020-0162. Epub 2020 Sep 2. Epigenomics. 2020. PMID: 32875809 Free PMC article. Review.
References
-
- Collins PLaC, JE . Field’s Virology. 5. 2007. Respiratory Syncytial Virus and Human Metapneumovirus; pp. 1601–46.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

