Modulation of Biofilm Exopolysaccharides by the Streptococcus mutans vicX Gene
- PMID: 26733973
- PMCID: PMC4685068
- DOI: 10.3389/fmicb.2015.01432
Modulation of Biofilm Exopolysaccharides by the Streptococcus mutans vicX Gene
Abstract
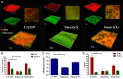
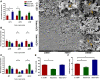
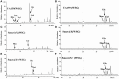
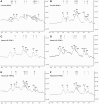
The cariogenic pathogen Streptococcus mutans effectively utilizes dietary sucrose for the synthesis of exopolysaccharide, which act as a scaffold for its biofilm, thus contributing to its pathogenicity, environmental stress tolerance, and antimicrobial resistance. The two-component system VicRK of S. mutans regulates a group of virulence genes that are associated with biofilm matrix synthesis. Knockout of vicX affects biofilm formation, oxidative stress tolerance, and transformation of S. mutans. However, little is known regarding the vicX-modulated structural characteristics of the exopolysaccharides underlying the biofilm formation and the phenotypes of the vicX mutants. Here, we identified the role of vicX in the structural characteristics of the exopolysaccharide matrix and biofilm physiology. The vicX mutant (SmuvicX) biofilms seemingly exhibited "desertification" with architecturally impaired exopolysaccharide-enmeshed cell clusters, compared with the UA159 strain (S. mutans wild type strain). Concomitantly, SmuvicX showed a decrease in water-insoluble glucan (WIG) synthesis and in WIG/water-soluble glucan (WSG) ratio. Gel permeation chromatography (GPC) showed that the WIG isolated from the SmuvicX biofilms had a much lower molecular weight compared with the UA159 strain indicating differences in polysaccharide chain lengths. A monosaccharide composition analysis demonstrated the importance of the vicX gene in the glucose metabolism. We performed metabolite profiling via (1)H nuclear magnetic resonance spectroscopy, which showed that several chemical shifts were absent in both WSG and WIG of SmuvicX biofilms compared with the UA159 strain. Thus, the modulation of structural characteristics of exopolysaccharide by vicX provides new insights into the interaction between the exopolysaccharide structure, gene functions, and cariogenicity. Our results suggest that vicX gene modulates the structural characteristics of exopolysaccharide associated with cariogenicity, which may be explored as a potential target that contributes to dental caries management. Furthermore, the methods used to purify the EPS of S. mutans biofilms and to analyze multiple aspects of its structure (GPC, gas chromatography-mass spectrometry, and (1)H nuclear magnetic resonance spectroscopy) may be useful approaches to determine the roles of other virulence genes for dental caries prevention.
Keywords: Streptococcus mutans; biofilms; caries prevention; glucosyltransferase; polysaccharides; two component VicRK system.
Figures

Similar articles
-
The Streptococcus mutans vicX gene product modulates gtfB/C expression, biofilm formation, genetic competence, and oxidative stress tolerance.J Bacteriol. 2007 Feb;189(4):1451-8. doi: 10.1128/JB.01161-06. Epub 2006 Nov 17. J Bacteriol. 2007. PMID: 17114248 Free PMC article.
-
Regulation of water-soluble glucan synthesis by the Streptococcus mutans dexA gene effects biofilm aggregation and cariogenic pathogenicity.Mol Oral Microbiol. 2019 Apr;34(2):51-63. doi: 10.1111/omi.12253. Epub 2019 Feb 14. Mol Oral Microbiol. 2019. PMID: 30659765
-
The rnc Gene Regulates the Microstructure of Exopolysaccharide in the Biofilm of Streptococcus mutans through the β-Monosaccharides.Caries Res. 2021;55(5):534-545. doi: 10.1159/000518462. Epub 2021 Jul 26. Caries Res. 2021. PMID: 34348276
-
Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms.Front Cell Infect Microbiol. 2015 Feb 13;5:10. doi: 10.3389/fcimb.2015.00010. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25763359 Free PMC article. Review.
-
The findings of glucosyltransferase enzymes derived from oral streptococci.Jpn Dent Sci Rev. 2022 Nov;58:328-335. doi: 10.1016/j.jdsr.2022.10.003. Epub 2022 Oct 28. Jpn Dent Sci Rev. 2022. PMID: 36340584 Free PMC article. Review.
Cited by
-
TetR Family Regulator brpT Modulates Biofilm Formation in Streptococcus sanguinis.PLoS One. 2017 Jan 3;12(1):e0169301. doi: 10.1371/journal.pone.0169301. eCollection 2017. PLoS One. 2017. PMID: 28046010 Free PMC article.
-
Capsular polysaccharide inhibits adhesion of Bifidobacterium longum 105-A to enterocyte-like Caco-2 cells and phagocytosis by macrophages.Gut Pathog. 2017 May 1;9:27. doi: 10.1186/s13099-017-0177-x. eCollection 2017. Gut Pathog. 2017. PMID: 28469711 Free PMC article.
-
Antimicrobial Effect of a Peptide Containing Novel Oral Spray on Streptococcus mutans.Biomed Res Int. 2020 Mar 10;2020:6853652. doi: 10.1155/2020/6853652. eCollection 2020. Biomed Res Int. 2020. PMID: 32258136 Free PMC article.
-
Oxidative Stressors Modify the Response of Streptococcus mutans to Its Competence Signal Peptides.Appl Environ Microbiol. 2017 Oct 31;83(22):e01345-17. doi: 10.1128/AEM.01345-17. Print 2017 Nov 15. Appl Environ Microbiol. 2017. PMID: 28887419 Free PMC article.
-
Arginine-induced metabolomic perturbation in Streptococcus mutans.J Oral Microbiol. 2022 Jan 7;14(1):2015166. doi: 10.1080/20002297.2021.2015166. eCollection 2022. J Oral Microbiol. 2022. PMID: 35024088 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

