Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy
- PMID: 26719539
- PMCID: PMC4829106
- DOI: 10.1158/0008-5472.CAN-15-1743
Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy
Erratum in
-
Correction: Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy.Cancer Res. 2017 May 1;77(9):2552. doi: 10.1158/0008-5472.CAN-17-0559. Cancer Res. 2017. PMID: 28461565 No abstract available.
Abstract
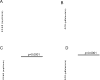
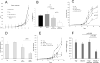
Cancer immunotherapies, such as immune checkpoint blockade or adoptive T-cell transfer, can lead to durable responses in the clinic, but response rates remain low due to undefined suppression mechanisms. Solid tumors are characterized by a highly acidic microenvironment that might blunt the effectiveness of antitumor immunity. In this study, we directly investigated the effects of tumor acidity on the efficacy of immunotherapy. An acidic pH environment blocked T-cell activation and limited glycolysis in vitro. IFNγ release blocked by acidic pH did not occur at the level of steady-state mRNA, implying that the effect of acidity was posttranslational. Acidification did not affect cytoplasmic pH, suggesting that signals transduced by external acidity were likely mediated by specific acid-sensing receptors, four of which are expressed by T cells. Notably, neutralizing tumor acidity with bicarbonate monotherapy impaired the growth of some cancer types in mice where it was associated with increased T-cell infiltration. Furthermore, combining bicarbonate therapy with anti-CTLA-4, anti-PD1, or adoptive T-cell transfer improved antitumor responses in multiple models, including cures in some subjects. Overall, our findings show how raising intratumoral pH through oral buffers therapy can improve responses to immunotherapy, with the potential for immediate clinical translation.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest: R.J. Gillies is shareholder in and member of scientific advisory board of Health-Myne, Inc. S. Pilon-Thomas is grant recipient from Lion Biotechnologies, Inc.
Figures
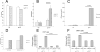



Similar articles
-
Poxvirus-based active immunotherapy synergizes with CTLA-4 blockade to increase survival in a murine tumor model by improving the magnitude and quality of cytotoxic T cells.Cancer Immunol Immunother. 2016 May;65(5):537-49. doi: 10.1007/s00262-016-1816-7. Epub 2016 Mar 10. Cancer Immunol Immunother. 2016. PMID: 26961085 Free PMC article.
-
Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer.Eur J Immunol. 2017 Feb;47(2):385-393. doi: 10.1002/eji.201646583. Epub 2016 Dec 13. Eur J Immunol. 2017. PMID: 27873300
-
TSC2-deficient tumors have evidence of T cell exhaustion and respond to anti-PD-1/anti-CTLA-4 immunotherapy.JCI Insight. 2018 Apr 19;3(8):e98674. doi: 10.1172/jci.insight.98674. eCollection 2018 Apr 19. JCI Insight. 2018. PMID: 29669930 Free PMC article.
-
Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity.Br J Haematol. 2013 Aug;162(3):313-25. doi: 10.1111/bjh.12380. Epub 2013 May 21. Br J Haematol. 2013. PMID: 23691926 Review.
-
Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors.Cytokine Growth Factor Rev. 2017 Aug;36:5-15. doi: 10.1016/j.cytogfr.2017.06.011. Epub 2017 Jun 28. Cytokine Growth Factor Rev. 2017. PMID: 28693973 Review.
Cited by
-
Back to basic: Trials and tribulations of alkalizing agents in cancer.Front Oncol. 2022 Nov 14;12:981718. doi: 10.3389/fonc.2022.981718. eCollection 2022. Front Oncol. 2022. PMID: 36452492 Free PMC article.
-
Proposal to Consider Chemical/Physical Microenvironment as a New Therapeutic Off-Target Approach.Pharmaceutics. 2022 Sep 29;14(10):2084. doi: 10.3390/pharmaceutics14102084. Pharmaceutics. 2022. PMID: 36297518 Free PMC article.
-
Glycolysis in tumor microenvironment as a target to improve cancer immunotherapy.Front Cell Dev Biol. 2022 Sep 19;10:1013885. doi: 10.3389/fcell.2022.1013885. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36200045 Free PMC article. Review.
-
Hydrogen Ion Dynamics of Cancer and a New Molecular, Biochemical and Metabolic Approach to the Etiopathogenesis and Treatment of Brain Malignancies.Int J Mol Sci. 2019 Sep 1;20(17):4278. doi: 10.3390/ijms20174278. Int J Mol Sci. 2019. PMID: 31480530 Free PMC article. Review.
-
A drug screening assay on cancer cells chronically adapted to acidosis.Cancer Cell Int. 2018 Sep 25;18:147. doi: 10.1186/s12935-018-0645-5. eCollection 2018. Cancer Cell Int. 2018. PMID: 30263014 Free PMC article.
References
-
- Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313(5795):1972–5. - PubMed
-
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual review of immunology. 2011;29:235–71. - PubMed
-
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(10):1020–30. - PMC - PubMed
-
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(19):5064–74. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

