Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers
- PMID: 26717006
- PMCID: PMC4696808
- DOI: 10.1371/journal.pone.0145754
Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers
Abstract
Background: High-grade serous ovarian and endometrial cancers are the most lethal female reproductive tract malignancies worldwide. In part, failure to treat these two aggressive cancers successfully centers on the fact that while the majority of patients are diagnosed based on current surveillance strategies as having a complete clinical response to their primary therapy, nearly half will develop disease recurrence within 18 months and the majority will die from disease recurrence within 5 years. Moreover, no currently used biomarkers or imaging studies can predict outcome following initial treatment. Circulating tumor DNA (ctDNA) represents a theoretically powerful biomarker for detecting otherwise occult disease. We therefore explored the use of personalized ctDNA markers as both a surveillance and prognostic biomarker in gynecologic cancers and compared this to current FDA-approved surveillance tools.
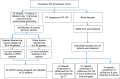
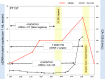
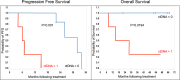
Methods and findings: Tumor and serum samples were collected at time of surgery and then throughout treatment course for 44 patients with gynecologic cancers, representing 22 ovarian cancer cases, 17 uterine cancer cases, one peritoneal, three fallopian tube, and one patient with synchronous fallopian tube and uterine cancer. Patient/tumor-specific mutations were identified using whole-exome and targeted gene sequencing and ctDNA levels quantified using droplet digital PCR. CtDNA was detected in 93.8% of patients for whom probes were designed and levels were highly correlated with CA-125 serum and computed tomography (CT) scanning results. In six patients, ctDNA detected the presence of cancer even when CT scanning was negative and, on average, had a predictive lead time of seven months over CT imaging. Most notably, undetectable levels of ctDNA at six months following initial treatment was associated with markedly improved progression free and overall survival.
Conclusions: Detection of residual disease in gynecologic, and indeed all cancers, represents a diagnostic dilemma and a potential critical inflection point in precision medicine. This study suggests that the use of personalized ctDNA biomarkers in gynecologic cancers can identify the presence of residual tumor while also more dynamically predicting response to treatment relative to currently used serum and imaging studies. Of particular interest, ctDNA was an independent predictor of survival in patients with ovarian and endometrial cancers. Earlier recognition of disease persistence and/or recurrence and the ability to stratify into better and worse outcome groups through ctDNA surveillance may open the window for improved survival and quality and life in these cancers.
Conflict of interest statement
Figures
Similar articles
-
Monitoring Treatment Response, Early Recurrence, and Survival in Uterine Serous Carcinoma and Carcinosarcoma Patients Using Personalized Circulating Tumor DNA Biomarkers.Int J Mol Sci. 2023 May 17;24(10):8873. doi: 10.3390/ijms24108873. Int J Mol Sci. 2023. PMID: 37240216 Free PMC article.
-
Circulating Tumor DNA (ctDNA) and Its Role in Gynecologic Malignancies.Curr Treat Options Oncol. 2024 Apr;25(4):510-522. doi: 10.1007/s11864-024-01180-w. Epub 2024 Mar 12. Curr Treat Options Oncol. 2024. PMID: 38472567 Review.
-
Prognostic significance of rising serum CA-125 levels within the normal range in patients with epithelial ovarian, primary peritoneal, and tubal cancers, who, after initial treatment, had a complete clinical response.Int J Gynecol Cancer. 2012 Oct;22(8):1344-8. doi: 10.1097/IGC.0b013e3182691254. Int J Gynecol Cancer. 2012. PMID: 22954785
-
Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study.PLoS Med. 2016 Dec 20;13(12):e1002198. doi: 10.1371/journal.pmed.1002198. eCollection 2016 Dec. PLoS Med. 2016. PMID: 27997533 Free PMC article.
-
The role of the HER-2/neu oncogene in gynecologic cancers.J Soc Gynecol Investig. 1996 May-Jun;3(3):99-105. J Soc Gynecol Investig. 1996. PMID: 8796816 Review.
Cited by
-
Computational models for predicting drug responses in cancer research.Brief Bioinform. 2017 Sep 1;18(5):820-829. doi: 10.1093/bib/bbw065. Brief Bioinform. 2017. PMID: 27444372 Free PMC article. Review.
-
Personalized tumor-specific DNA junctions to detect circulating tumor in patients with endometrial cancer.PLoS One. 2021 Jun 10;16(6):e0252390. doi: 10.1371/journal.pone.0252390. eCollection 2021. PLoS One. 2021. PMID: 34111149 Free PMC article.
-
Evaluating the Utility of ctDNA in Detecting Residual Cancer and Predicting Recurrence in Patients with Serous Ovarian Cancer.Int J Mol Sci. 2023 Sep 21;24(18):14388. doi: 10.3390/ijms241814388. Int J Mol Sci. 2023. PMID: 37762691 Free PMC article.
-
Non-invasive Technology Advances in Cancer-A Review of the Advances in the Liquid Biopsy for Endometrial and Ovarian Cancers.Front Digit Health. 2020 Dec 11;2:573010. doi: 10.3389/fdgth.2020.573010. eCollection 2020. Front Digit Health. 2020. PMID: 34713045 Free PMC article. Review.
-
Clinical relevance of circulating ESR1 mutations during endocrine therapy for advanced hormone-dependent endometrial carcinoma.BMC Cancer. 2023 Nov 3;23(1):1061. doi: 10.1186/s12885-023-11559-x. BMC Cancer. 2023. PMID: 37924026 Free PMC article.
References
-
- American Cancer Society. Cancer Facts & Figs 2015 [Internet]. Atlanta: American Cancer Society; 2015. Available: http://www.cancer.org/acs/groups/content/@editorial/documents/document/a.... Accessed 1 September 2015.
-
- Ueland FR, DePriest PD, Pavlik EJ, Kryscio RJ, van Nagell JR. Preoperative differentiation of malignant from benign ovarian tumors: the efficacy of morphology indexing and Doppler flow sonography. Gynecol Oncol. 2003. October;91(1):46–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

