Pregnancy Outcomes after a Mass Vaccination Campaign with an Oral Cholera Vaccine in Guinea: A Retrospective Cohort Study
- PMID: 26713614
- PMCID: PMC4695076
- DOI: 10.1371/journal.pntd.0004274
Pregnancy Outcomes after a Mass Vaccination Campaign with an Oral Cholera Vaccine in Guinea: A Retrospective Cohort Study
Abstract
Introduction: Since 2010, WHO has recommended oral cholera vaccines as an additional strategy for cholera control. During a cholera episode, pregnant women are at high risk of complications, and the risk of fetal death has been reported to be 2-36%. Due to a lack of safety data, pregnant women have been excluded from most cholera vaccination campaigns. In 2012, reactive campaigns using the bivalent killed whole-cell oral cholera vaccine (BivWC), included all people living in the targeted areas aged ≥ 1 year regardless of pregnancy status, were implemented in Guinea. We aimed to determine whether there was a difference in pregnancy outcomes between vaccinated and non-vaccinated pregnant women.
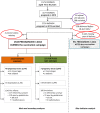
Methods and findings: From 11 November to 4 December 2013, we conducted a retrospective cohort study in Boffa prefecture among women who were pregnant in 2012 during or after the vaccination campaign. The primary outcome was pregnancy loss, as reported by the mother, and fetal malformations, after clinical examination. Primary exposure was the intake of the BivWC vaccine (Shanchol) during pregnancy, as determined by a vaccination card or oral history. We compared the risk of pregnancy loss between vaccinated and non-vaccinated women through binomial regression analysis. A total of 2,494 pregnancies were included in the analysis. The crude incidence of pregnancy loss was 3.7% (95%CI 2.7-4.8) for fetuses exposed to BivWC vaccine and 2.6% (0.7-4.5) for non-exposed fetuses. The incidence of malformation was 0.6% (0.1-1.0) and 1.2% (0.0-2.5) in BivWC-exposed and non-exposed fetuses, respectively. In both crude and adjusted analyses, fetal exposure to BivWC was not significantly associated with pregnancy loss (adjusted risk ratio (aRR = 1.09 [95%CI: 0.5-2.25], p = 0.818) or malformations (aRR = 0.50 [95%CI: 0.13-1.91], p = 0.314).
Conclusions: In this large retrospective cohort study, we found no association between fetal exposure to BivWC and risk of pregnancy loss or malformation. Despite the weaknesses of a retrospective design, we can conclude that if a risk exists, it is very low. Additional prospective studies are warranted to add to the evidence base on OCV use during pregnancy. Pregnant women are particularly vulnerable during cholera episodes and should be included in vaccination campaigns when the risk of cholera is high, such as during outbreaks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Safety of a killed oral cholera vaccine (Shanchol) in pregnant women in Malawi: an observational cohort study.Lancet Infect Dis. 2017 May;17(5):538-544. doi: 10.1016/S1473-3099(16)30523-0. Epub 2017 Feb 2. Lancet Infect Dis. 2017. PMID: 28161570 Free PMC article. Clinical Trial.
-
Safety of a bivalent, killed, whole-cell oral cholera vaccine in pregnant women in Bangladesh: evidence from a randomized placebo-controlled trial.BMC Infect Dis. 2019 May 15;19(1):422. doi: 10.1186/s12879-019-4006-3. BMC Infect Dis. 2019. PMID: 31092224 Free PMC article. Clinical Trial.
-
Safety of the oral cholera vaccine in pregnancy: Retrospective findings from a subgroup following mass vaccination campaign in Dhaka, Bangladesh.Vaccine. 2017 Mar 13;35(11):1538-1543. doi: 10.1016/j.vaccine.2017.01.080. Epub 2017 Feb 11. Vaccine. 2017. PMID: 28196715 Free PMC article.
-
Oral cholera vaccination coverage in an acute emergency setting in Somalia, 2017.Vaccine. 2020 Feb 29;38 Suppl 1:A141-A147. doi: 10.1016/j.vaccine.2020.01.015. Epub 2020 Jan 21. Vaccine. 2020. PMID: 31980193 Review.
-
Comprehensive Review on the Use of Oral Cholera Vaccine (OCV) in Ethiopia: 2019 to 2023.Clin Infect Dis. 2024 Jul 12;79(Supplement_1):S20-S32. doi: 10.1093/cid/ciae194. Clin Infect Dis. 2024. PMID: 38996040 Free PMC article. Review.
Cited by
-
Safety of a killed oral cholera vaccine (Shanchol) in pregnant women in Malawi: an observational cohort study.Lancet Infect Dis. 2017 May;17(5):538-544. doi: 10.1016/S1473-3099(16)30523-0. Epub 2017 Feb 2. Lancet Infect Dis. 2017. PMID: 28161570 Free PMC article. Clinical Trial.
-
Progress and Challenges in Using Oral Cholera Vaccines to Control Outbreaks: The Médecins Sans Frontières Experience.J Infect Dis. 2018 Oct 15;218(suppl_3):S165-S166. doi: 10.1093/infdis/jiy487. J Infect Dis. 2018. PMID: 30239901 Free PMC article.
-
Diagnosis, Management, and Future Control of Cholera.Clin Microbiol Rev. 2022 Sep 21;35(3):e0021121. doi: 10.1128/cmr.00211-21. Epub 2022 Jun 21. Clin Microbiol Rev. 2022. PMID: 35726607 Free PMC article. Review.
-
Safety of oral cholera vaccines during pregnancy in developing countries.Hum Vaccin Immunother. 2017 Oct 3;13(10):2245-2246. doi: 10.1080/21645515.2017.1356525. Hum Vaccin Immunother. 2017. PMID: 28825876 Free PMC article. No abstract available.
-
Licensed and Recommended Inactivated Oral CholeraVaccines: From Development to Innovative Deployment.Trop Med Infect Dis. 2021 Mar 9;6(1):32. doi: 10.3390/tropicalmed6010032. Trop Med Infect Dis. 2021. PMID: 33803390 Free PMC article. Review.
References
-
- Khan PK. Asiatic cholera in pregnancy. Int Surg. 1969; 51: 138–141. - PubMed
-
- Hirschhorn N, Chowdhury AK, Lindenbaum J. Cholera in pregnant women. Lancet. 1969; 1: 1230–1232. - PubMed
-
- Ayangade O. The significance of cholera outbreak in the prognosis of pregnancy. Int J Gynaecol Obstet. 1981; 19: 403–407. - PubMed
-
- Saona P, Astudillo J, Figueroa M, Maradiegue E. Cholera in pregnant women at the Hospital Nacional Cayetano Heredia, Lima Peru. Rev Med Hered. 1981; 2: 112–116.
-
- Grados P, Batillana C. El traitamento de la diarhea coleriforme en la gestacion. Bol of Sanit Panam. 1994; 116: 198–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical