Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial
- PMID: 26707054
- PMCID: PMC4779792
- DOI: 10.1016/S0140-6736(15)01224-6
Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial
Erratum in
- Lancet. 2016 Mar 5;387(10022):944
-
Department of Error.Lancet. 2016 Mar 5;387(10022):944. doi: 10.1016/S0140-6736(16)00228-2. Epub 2016 Jan 30. Lancet. 2016. PMID: 28832000 Free PMC article. No abstract available.
Abstract
Background: Ovarian cancer has a poor prognosis, with just 40% of patients surviving 5 years. We designed this trial to establish the effect of early detection by screening on ovarian cancer mortality.
Methods: In this randomised controlled trial, we recruited postmenopausal women aged 50-74 years from 13 centres in National Health Service Trusts in England, Wales, and Northern Ireland. Exclusion criteria were previous bilateral oophorectomy or ovarian malignancy, increased risk of familial ovarian cancer, and active non-ovarian malignancy. The trial management system confirmed eligibility and randomly allocated participants in blocks of 32 using computer-generated random numbers to annual multimodal screening (MMS) with serum CA125 interpreted with use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound screening (USS), or no screening, in a 1:1:2 ratio. The primary outcome was death due to ovarian cancer by Dec 31, 2014, comparing MMS and USS separately with no screening, ascertained by an outcomes committee masked to randomisation group. All analyses were by modified intention to screen, excluding the small number of women we discovered after randomisation to have a bilateral oophorectomy, have ovarian cancer, or had exited the registry before recruitment. Investigators and participants were aware of screening type. This trial is registered with ClinicalTrials.gov, number NCT00058032.
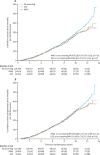
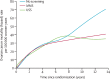
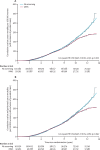
Findings: Between June 1, 2001, and Oct 21, 2005, we randomly allocated 202,638 women: 50,640 (25·0%) to MMS, 50,639 (25·0%) to USS, and 101,359 (50·0%) to no screening. 202,546 (>99·9%) women were eligible for analysis: 50,624 (>99·9%) women in the MMS group, 50,623 (>99·9%) in the USS group, and 101,299 (>99·9%) in the no screening group. Screening ended on Dec 31, 2011, and included 345,570 MMS and 327,775 USS annual screening episodes. At a median follow-up of 11·1 years (IQR 10·0-12·0), we diagnosed ovarian cancer in 1282 (0·6%) women: 338 (0·7%) in the MMS group, 314 (0·6%) in the USS group, and 630 (0·6%) in the no screening group. Of these women, 148 (0·29%) women in the MMS group, 154 (0·30%) in the USS group, and 347 (0·34%) in the no screening group had died of ovarian cancer. The primary analysis using a Cox proportional hazards model gave a mortality reduction over years 0-14 of 15% (95% CI -3 to 30; p=0·10) with MMS and 11% (-7 to 27; p=0·21) with USS. The Royston-Parmar flexible parametric model showed that in the MMS group, this mortality effect was made up of 8% (-20 to 31) in years 0-7 and 23% (1-46) in years 7-14, and in the USS group, of 2% (-27 to 26) in years 0-7 and 21% (-2 to 42) in years 7-14. A prespecified analysis of death from ovarian cancer of MMS versus no screening with exclusion of prevalent cases showed significantly different death rates (p=0·021), with an overall average mortality reduction of 20% (-2 to 40) and a reduction of 8% (-27 to 43) in years 0-7 and 28% (-3 to 49) in years 7-14 in favour of MMS.
Interpretation: Although the mortality reduction was not significant in the primary analysis, we noted a significant mortality reduction with MMS when prevalent cases were excluded. We noted encouraging evidence of a mortality reduction in years 7-14, but further follow-up is needed before firm conclusions can be reached on the efficacy and cost-effectiveness of ovarian cancer screening.
Funding: Medical Research Council, Cancer Research UK, Department of Health, The Eve Appeal.
Copyright © 2016 Jacobs Menon et al. Open Access article published under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures
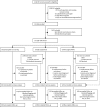
Comment in
-
Whether screening for ovarian cancer saves lives is still unclear despite large trial.BMJ. 2015 Dec 18;351:h6876. doi: 10.1136/bmj.h6876. BMJ. 2015. PMID: 26684480 No abstract available.
-
Screening to improve ovarian cancer prognosis?Lancet. 2016 Mar 5;387(10022):921-923. doi: 10.1016/S0140-6736(15)01236-2. Epub 2015 Dec 17. Lancet. 2016. PMID: 26707055 No abstract available.
-
Ovarian cancer: Multimodal screening - keeping mortality at bay?Nat Rev Clin Oncol. 2016 Mar;13(3):133. doi: 10.1038/nrclinonc.2016.10. Epub 2016 Jan 27. Nat Rev Clin Oncol. 2016. PMID: 26813936 No abstract available.
-
Ovarian Cancer Screening There May Be Light at the End of the Tunnel?Int J Gynecol Cancer. 2016 May;26(4):608-9. doi: 10.1097/IGC.0000000000000706. Int J Gynecol Cancer. 2016. PMID: 27101522 No abstract available.
-
Two large randomised trials show ovarian cancer screening has minimal impact on survival.BJOG. 2018 Apr;125(5):524-525. doi: 10.1111/1471-0528.14052. Epub 2016 May 13. BJOG. 2018. PMID: 27173436 No abstract available.
-
Ovarian cancer screening: UKCTOCS trial.Lancet. 2016 Jun 25;387(10038):2601-2602. doi: 10.1016/S0140-6736(16)30846-7. Lancet. 2016. PMID: 27353818 No abstract available.
-
Ovarian cancer screening: UKCTOCS trial.Lancet. 2016 Jun 25;387(10038):2602. doi: 10.1016/S0140-6736(16)30847-9. Lancet. 2016. PMID: 27353820 No abstract available.
-
Ovarian cancer screening: UKCTOCS trial.Lancet. 2016 Jun 25;387(10038):2602-2603. doi: 10.1016/S0140-6736(16)30848-0. Lancet. 2016. PMID: 27353821 No abstract available.
-
Ovarian cancer screening: UKCTOCS trial - Authors' reply.Lancet. 2016 Jun 25;387(10038):2603-2604. doi: 10.1016/S0140-6736(16)30849-2. Lancet. 2016. PMID: 27353822 No abstract available.
-
Large ovarian cancer screening trial shows modest mortality reduction, but does not justify population-based ovarian cancer screening.Evid Based Med. 2016 Aug;21(4):159. doi: 10.1136/ebmed-2016-110411. Epub 2016 Jul 22. Evid Based Med. 2016. PMID: 27450366 No abstract available.
-
Ovarian cancer screening effectiveness: A realization from the UK Collaborative Trial of Ovarian Cancer Screening.Womens Health (Lond). 2016 Sep;12(5):475-479. doi: 10.1177/1745505716666096. Epub 2016 Sep 5. Womens Health (Lond). 2016. PMID: 27595999 Free PMC article.
Similar articles
-
Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial.Lancet. 2021 Jun 5;397(10290):2182-2193. doi: 10.1016/S0140-6736(21)00731-5. Epub 2021 May 12. Lancet. 2021. PMID: 33991479 Free PMC article. Clinical Trial.
-
Mortality impact, risks, and benefits of general population screening for ovarian cancer: the UKCTOCS randomised controlled trial.Health Technol Assess. 2023 May 11:1-81. doi: 10.3310/BHBR5832. Online ahead of print. Health Technol Assess. 2023. PMID: 37183782 Free PMC article.
-
Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS).Lancet Oncol. 2009 Apr;10(4):327-40. doi: 10.1016/S1470-2045(09)70026-9. Epub 2009 Mar 11. Lancet Oncol. 2009. PMID: 19282241 Clinical Trial.
-
Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2018 Feb 13;319(6):595-606. doi: 10.1001/jama.2017.21421. JAMA. 2018. PMID: 29450530 Review.
-
The role of transvaginal ultrasound in screening for ovarian cancer.Climacteric. 2018 Jun;21(3):221-226. doi: 10.1080/13697137.2018.1433656. Epub 2018 Mar 1. Climacteric. 2018. PMID: 29490504 Review.
Cited by
-
Radiologic-Histopathologic Correlation of Transvaginal US and Risk-reducing Salpingo-oophorectomy for Women at High Risk for Tubo-ovarian Carcinoma.Radiol Imaging Cancer. 2020 Nov 13;2(6):e190086. doi: 10.1148/rycan.2020190086. eCollection 2020 Nov. Radiol Imaging Cancer. 2020. PMID: 33778746 Free PMC article.
-
Systematic review and meta-analysis of studies assessing the relationship between statin use and risk of ovarian cancer.Cancer Causes Control. 2020 Oct;31(10):869-879. doi: 10.1007/s10552-020-01327-8. Epub 2020 Jul 20. Cancer Causes Control. 2020. PMID: 32685996 Free PMC article.
-
Direct access from general practice to transvaginal ultrasound for early detection of ovarian cancer: a feasibility study.Scand J Prim Health Care. 2021 Jun;39(2):230-239. doi: 10.1080/02813432.2021.1922831. Epub 2021 Jun 7. Scand J Prim Health Care. 2021. PMID: 34092179 Free PMC article.
-
[Adnexal Masses: Clinical Application of Multiparametric MR Imaging & O-RADS MRI].Taehan Yongsang Uihakhoe Chi. 2021 Sep;82(5):1066-1082. doi: 10.3348/jksr.2021.0111. Epub 2021 Sep 15. Taehan Yongsang Uihakhoe Chi. 2021. PMID: 36238388 Free PMC article. Review. Korean.
-
New paradigms for BRCA1/BRCA2 testing in women with ovarian cancer: results of the Genetic Testing in Epithelial Ovarian Cancer (GTEOC) study.J Med Genet. 2016 Oct;53(10):655-61. doi: 10.1136/jmedgenet-2016-103902. Epub 2016 May 12. J Med Genet. 2016. PMID: 27208206 Free PMC article.
References
-
- Cancer Research UK. Ovarian cancer survival statistics. One-, five- and ten-year survival for ovarian cancer. http://www.cancerresearchuk.org/health-professional/cancer-statistics/st... (accessed Nov 3, 2015)
-
- Jacobs I, Stabile I, Bridges J, et al. Multimodal approach to screening for ovarian cancer. Lancet. 1988;1:268–271. - PubMed
-
- Jacobs IJ, Skates SJ, MacDonald N, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353:1207–1210. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

