Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI
- PMID: 26699510
- PMCID: PMC4711859
- DOI: 10.1073/pnas.1522297113
Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI
Abstract
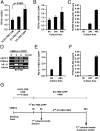
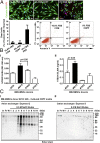
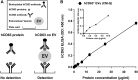
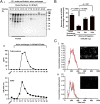
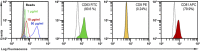
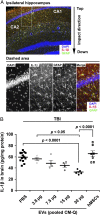
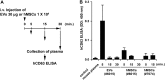
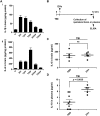
Extracellular vesicles (EVs) secreted by cells present an attractive strategy for developing new therapies, but progress in the field is limited by several issues: The quality of the EVs varies with the type and physiological status of the producer cells; protocols used to isolate the EVs are difficult to scale up; and assays for efficacy are difficult to develop. In the present report, we have addressed these issues by using human mesenchymal stem/stromal cells (MSCs) that produce EVs when incubated in a protein-free medium, preselecting the preparations of MSCs with a biomarker for their potency in modulating inflammation, incubating the cells in a chemically defined protein-free medium that provided a stable environment, isolating the EVs with a scalable chromatographic procedure, and developing an in vivo assay for efficacy of the cells in suppressing neuroinflammation after traumatic brain injury (TBI) in mice. In addition, we demonstrate that i.v. infusion of the isolated EVs shortly after induction of TBI rescued pattern separation and spatial learning impairments 1 mo later.
Keywords: MSCs; efficacy assay; exosomes; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats.J Neuroinflammation. 2019 Nov 13;16(1):216. doi: 10.1186/s12974-019-1602-5. J Neuroinflammation. 2019. PMID: 31722731 Free PMC article.
-
Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles.Sci Rep. 2019 Aug 9;9(1):11584. doi: 10.1038/s41598-019-48095-3. Sci Rep. 2019. PMID: 31399634 Free PMC article.
-
Individually cultured bovine embryos produce extracellular vesicles that have the potential to be used as non-invasive embryo quality markers.Theriogenology. 2020 Jun;149:104-116. doi: 10.1016/j.theriogenology.2020.03.008. Epub 2020 Apr 4. Theriogenology. 2020. PMID: 32259747
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury.Int J Mol Sci. 2021 Jun 20;22(12):6596. doi: 10.3390/ijms22126596. Int J Mol Sci. 2021. PMID: 34202940 Free PMC article. Review.
Cited by
-
Exosomes derived from MSC pre-treated with oridonin alleviates myocardial IR injury by suppressing apoptosis via regulating autophagy activation.J Cell Mol Med. 2021 Jun;25(12):5486-5496. doi: 10.1111/jcmm.16558. Epub 2021 May 6. J Cell Mol Med. 2021. PMID: 33955654 Free PMC article.
-
Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders.Neural Regen Res. 2021 Dec;16(12):2359-2366. doi: 10.4103/1673-5374.313026. Neural Regen Res. 2021. PMID: 33907007 Free PMC article. Review.
-
Phagocytosing differentiated cell-fragments is a novel mechanism for controlling somatic stem cell differentiation within a short time frame.Cell Mol Life Sci. 2022 Oct 6;79(11):542. doi: 10.1007/s00018-022-04555-0. Cell Mol Life Sci. 2022. PMID: 36203068 Free PMC article.
-
Promise of mesenchymal stem cell-derived extracellular vesicles for alleviating subarachnoid hemorrhage-induced brain dysfunction by neuroprotective and antiinflammatory effects.Brain Behav Immun Health. 2024 Aug 3;40:100835. doi: 10.1016/j.bbih.2024.100835. eCollection 2024 Oct. Brain Behav Immun Health. 2024. PMID: 39165307 Free PMC article. Review.
-
Cell-Derived Exosomes as Therapeutic Strategies and Exosome-Derived microRNAs as Biomarkers for Traumatic Brain Injury.J Clin Med. 2022 Jun 5;11(11):3223. doi: 10.3390/jcm11113223. J Clin Med. 2022. PMID: 35683610 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

