In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer
- PMID: 26689376
- PMCID: PMC4777632
- DOI: 10.1038/nnano.2015.292
In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer
Abstract
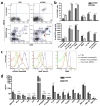
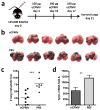
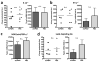
Nanotechnology has tremendous potential to contribute to cancer immunotherapy. The 'in situ vaccination' immunotherapy strategy directly manipulates identified tumours to overcome local tumour-mediated immunosuppression and subsequently stimulates systemic antitumour immunity to treat metastases. We show that inhalation of self-assembling virus-like nanoparticles from cowpea mosaic virus (CPMV) reduces established B16F10 lung melanoma and simultaneously generates potent systemic antitumour immunity against poorly immunogenic B16F10 in the skin. Full efficacy required Il-12, Ifn-γ, adaptive immunity and neutrophils. Inhaled CPMV nanoparticles were rapidly taken up by and activated neutrophils in the tumour microenvironment as an important part of the antitumour immune response. CPMV also exhibited clear treatment efficacy and systemic antitumour immunity in ovarian, colon, and breast tumour models in multiple anatomic locations. CPMV nanoparticles are stable, nontoxic, modifiable with drugs and antigens, and their nanomanufacture is highly scalable. These properties, combined with their inherent immunogenicity and demonstrated efficacy against a poorly immunogenic tumour, make CPMV an attractive and novel immunotherapy against metastatic cancer.
Conflict of interest statement
P.H.L., A.M.W., N.F.S., and S.F. have applied for patent protection for the immunotherapeutic use of eCPMV.
Figures
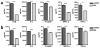

Comment in
-
Cancer immunotherapy: A vaccine from plant virus proteins.Nat Nanotechnol. 2016 Mar;11(3):214-5. doi: 10.1038/nnano.2015.306. Epub 2015 Dec 21. Nat Nanotechnol. 2016. PMID: 26689377 No abstract available.
Similar articles
-
In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma.Mol Pharm. 2018 Sep 4;15(9):3700-3716. doi: 10.1021/acs.molpharmaceut.8b00316. Epub 2018 May 25. Mol Pharm. 2018. PMID: 29798673 Free PMC article.
-
Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties.J Virol. 2019 Oct 15;93(21):e00129-19. doi: 10.1128/JVI.00129-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375592 Free PMC article.
-
Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer.ACS Nano. 2020 Mar 24;14(3):2994-3003. doi: 10.1021/acsnano.9b07865. Epub 2020 Mar 5. ACS Nano. 2020. PMID: 32133838 Free PMC article.
-
Cowpea mosaic virus nanoparticles for cancer imaging and therapy.Adv Drug Deliv Rev. 2019 May;145:130-144. doi: 10.1016/j.addr.2019.04.005. Epub 2019 Apr 17. Adv Drug Deliv Rev. 2019. PMID: 31004625 Review.
-
Cowpea mosaic virus as a vaccine carrier of heterologous antigens.Mol Biotechnol. 2001 Jan;17(1):15-26. doi: 10.1385/MB:17:1:15. Mol Biotechnol. 2001. PMID: 11280928 Review.
Cited by
-
A bibliometric insight into nanomaterials in vaccine: trends, collaborations, and future avenues.Front Immunol. 2024 Aug 12;15:1420216. doi: 10.3389/fimmu.2024.1420216. eCollection 2024. Front Immunol. 2024. PMID: 39188723 Free PMC article.
-
Nanoparticles for imaging and treatment of metastatic breast cancer.Expert Opin Drug Deliv. 2017 Jan;14(1):123-136. doi: 10.1080/17425247.2016.1208650. Epub 2016 Jul 19. Expert Opin Drug Deliv. 2017. PMID: 27401941 Free PMC article. Review.
-
The Landscape of Nanovectors for Modulation in Cancer Immunotherapy.Pharmaceutics. 2022 Feb 11;14(2):397. doi: 10.3390/pharmaceutics14020397. Pharmaceutics. 2022. PMID: 35214129 Free PMC article. Review.
-
Protein nanoparticles with ligand-binding and enzymatic activities.Int J Nanomedicine. 2018 Oct 18;13:6637-6646. doi: 10.2147/IJN.S177627. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30425479 Free PMC article.
-
Nanoparticle systems for cancer vaccine.Nanomedicine (Lond). 2019 Mar;14(5):627-648. doi: 10.2217/nnm-2018-0147. Epub 2019 Feb 26. Nanomedicine (Lond). 2019. PMID: 30806568 Free PMC article. Review.
References
-
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13:525–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

