Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors
- PMID: 26684252
- PMCID: PMC4773846
- DOI: 10.1152/ajplung.00398.2015
Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors
Abstract
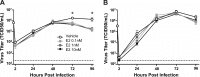
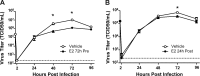
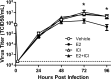
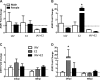
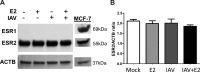
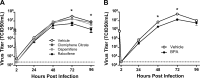
Influenza causes an acute infection characterized by virus replication in respiratory epithelial cells. The severity of influenza and other respiratory diseases changes over the life course and during pregnancy in women, suggesting that sex steroid hormones, such as estrogens, may be involved. Using primary, differentiated human nasal epithelial cell (hNEC) cultures from adult male and female donors, we exposed cultures to the endogenous 17β-estradiol (E2) or select estrogen receptor modulators (SERMs) and then infected cultures with a seasonal influenza A virus (IAV) to determine whether estrogenic signaling could affect the outcome of IAV infection and whether these effects were sex dependent. Estradiol, raloxifene, and bisphenol A decreased IAV titers in hNECs from female, but not male, donors. The estrogenic decrease in viral titer was dependent on the genomic estrogen receptor-2 (ESR2) as neither genomic ESR1 nor nongenomic GPR30 was expressed in hNEC cultures and addition of the genomic ER antagonist ICI 182,780 reversed the antiviral effects of E2. Treatment of hNECs with E2 had no effect on interferon or chemokine secretion but significantly downregulated cell metabolic processes, including genes that encode for zinc finger proteins, many of which contain estrogen response elements in their promoters. These data provide novel insights into the cellular and molecular mechanisms of how natural and synthetic estrogens impact IAV infection in respiratory epithelial cells derived from humans.
Keywords: SERMs; antiviral defenses; estradiol; respiratory disease; zinc finger proteins.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
Innate Immune Response to Influenza Virus at Single-Cell Resolution in Human Epithelial Cells Revealed Paracrine Induction of Interferon Lambda 1.J Virol. 2019 Sep 30;93(20):e00559-19. doi: 10.1128/JVI.00559-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375585 Free PMC article.
-
CD151, a novel host factor of nuclear export signaling in influenza virus infection.J Allergy Clin Immunol. 2018 May;141(5):1799-1817. doi: 10.1016/j.jaci.2017.11.032. Epub 2017 Dec 21. J Allergy Clin Immunol. 2018. PMID: 29274410
-
Vitamin D modulation of the activity of estrogenic compounds in bone cells in vitro and in vivo.Crit Rev Eukaryot Gene Expr. 2007;17(2):115-47. doi: 10.1615/critreveukargeneexpr.v17.i2.30. Crit Rev Eukaryot Gene Expr. 2007. PMID: 17725484 Review.
-
Restricted replication of the live attenuated influenza A virus vaccine during infection of primary differentiated human nasal epithelial cells.Vaccine. 2015 Aug 26;33(36):4495-504. doi: 10.1016/j.vaccine.2015.07.023. Epub 2015 Jul 18. Vaccine. 2015. PMID: 26196325 Free PMC article.
-
Influenza A virus interactions with macrophages: Lessons from epithelial cells.Cell Microbiol. 2020 May;22(5):e13170. doi: 10.1111/cmi.13170. Epub 2020 Feb 18. Cell Microbiol. 2020. PMID: 31990121 Review.
Cited by
-
Retrospective Analysis of the Effect of Postmenopausal Women Medications on SARS-CoV-2 Infection Progression.Life (Basel). 2024 Sep 3;14(9):1107. doi: 10.3390/life14091107. Life (Basel). 2024. PMID: 39337891 Free PMC article.
-
Association between receiving Covid-19 vaccine and menstrual cycle patterns among childbearing women: A cross-sectional study.Health Sci Rep. 2024 May 9;7(5):e1934. doi: 10.1002/hsr2.1934. eCollection 2024 May. Health Sci Rep. 2024. PMID: 38736480 Free PMC article.
-
Structure-Activity Relationships of Triphenylethylene Derivatives and Their Evaluation as Anticancer and Antiviral Agents.ACS Omega. 2023 Jul 11;8(29):25903-25923. doi: 10.1021/acsomega.3c01682. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521647 Free PMC article.
-
Characteristics of re-admitted adult patients infected with severe acute respiratory syndrome Coronavirus 2 After initial hospitalization at King Salman Armed Forces Hospital, Tabuk, Saudi Arabia.Saudi Med J. 2023 Jul;44(7):647-654. doi: 10.15537/smj.2023.44.7.20220713. Saudi Med J. 2023. PMID: 37463710 Free PMC article.
-
Tocilizumab-coated solid lipid nanoparticles loaded with cannabidiol as a novel drug delivery strategy for treating COVID-19: A review.Front Immunol. 2023 Mar 22;14:1147991. doi: 10.3389/fimmu.2023.1147991. eCollection 2023. Front Immunol. 2023. PMID: 37033914 Free PMC article. Review.
References
-
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138: 4613–4621, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

