Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation
- PMID: 26677765
- PMCID: PMC4682208
- DOI: 10.1016/j.stemcr.2015.10.014
Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation
Abstract
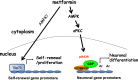
The recruitment of endogenous adult neural stem cells for brain repair is a promising regenerative therapeutic strategy. This strategy involves stimulation of multiple stages of adult neural stem cell development, including proliferation, self-renewal, and differentiation. Currently, there is a lack of a single therapeutic approach that can act on these multiple stages of adult neural stem cell development to enhance neural regeneration. Here we show that metformin, an FDA-approved diabetes drug, promotes proliferation, self-renewal, and differentiation of adult neural precursors (NPCs). Specifically, we show that metformin enhances adult NPC proliferation and self-renewal dependent upon the p53 family member and transcription factor TAp73, while it promotes neuronal differentiation of these cells by activating the AMPK-aPKC-CBP pathway. Thus, metformin represents an optimal candidate neuro-regenerative agent that is capable of not only expanding the adult NPC population but also subsequently driving them toward neuronal differentiation by activating two distinct molecular pathways.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Dysregulated expression of monoacylglycerol lipase is a marker for anti-diabetic drug metformin-targeted therapy to correct impaired neurogenesis and spatial memory in Alzheimer's disease.Theranostics. 2020 May 15;10(14):6337-6360. doi: 10.7150/thno.44962. eCollection 2020. Theranostics. 2020. PMID: 32483456 Free PMC article.
-
Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation.Cell Stem Cell. 2012 Jul 6;11(1):23-35. doi: 10.1016/j.stem.2012.03.016. Cell Stem Cell. 2012. PMID: 22770240
-
Metformin enhances neural precursor cells migration and functional recovery after ischemic stroke in mice.Exp Brain Res. 2023 Feb;241(2):505-515. doi: 10.1007/s00221-023-06547-3. Epub 2023 Jan 8. Exp Brain Res. 2023. PMID: 36611122
-
Effects of addictive drugs on adult neural stem/progenitor cells.Cell Mol Life Sci. 2016 Jan;73(2):327-48. doi: 10.1007/s00018-015-2067-z. Epub 2015 Oct 14. Cell Mol Life Sci. 2016. PMID: 26468052 Free PMC article. Review.
-
Immunopharmacological intervention for successful neural stem cell therapy: New perspectives in CNS neurogenesis and repair.Pharmacol Ther. 2014 Jan;141(1):21-31. doi: 10.1016/j.pharmthera.2013.08.001. Epub 2013 Aug 15. Pharmacol Ther. 2014. PMID: 23954656 Review.
Cited by
-
Neurogenic effects of rotarod walking exercise in subventricular zone, subgranular zone, and substantia nigra in MPTP-induced Parkinson's disease mice.Sci Rep. 2022 Jun 22;12(1):10544. doi: 10.1038/s41598-022-14823-5. Sci Rep. 2022. PMID: 35732806 Free PMC article.
-
Adverse Effects of Metformin From Diabetes to COVID-19, Cancer, Neurodegenerative Diseases, and Aging: Is VDAC1 a Common Target?Front Physiol. 2021 Oct 4;12:730048. doi: 10.3389/fphys.2021.730048. eCollection 2021. Front Physiol. 2021. PMID: 34671273 Free PMC article. Review.
-
Dysregulated expression of monoacylglycerol lipase is a marker for anti-diabetic drug metformin-targeted therapy to correct impaired neurogenesis and spatial memory in Alzheimer's disease.Theranostics. 2020 May 15;10(14):6337-6360. doi: 10.7150/thno.44962. eCollection 2020. Theranostics. 2020. PMID: 32483456 Free PMC article.
-
Mitochondrial and Autophagic Regulation of Adult Neurogenesis in the Healthy and Diseased Brain.Int J Mol Sci. 2021 Mar 24;22(7):3342. doi: 10.3390/ijms22073342. Int J Mol Sci. 2021. PMID: 33805219 Free PMC article. Review.
-
Metformin pretreatment rescues olfactory memory associated with subependymal zone neurogenesis in a juvenile model of cranial irradiation.Cell Rep Med. 2021 Apr 6;2(4):100231. doi: 10.1016/j.xcrm.2021.100231. eCollection 2021 Apr 20. Cell Rep Med. 2021. PMID: 33948569 Free PMC article.
References
-
- Engelmann D., Meier C., Alla V., Pützer B.M. A balancing act: orchestrating amino-truncated and full-length p73 variants as decisive factors in cancer progression. Oncogene. 2015;34:4287–4299. - PubMed
-
- Fujitani M., Cancino G.I., Dugani C.B., Weaver I.C., Gauthier-Fisher A., Paquin A., Mak T.W., Wojtowicz M.J., Miller F.D., Kaplan D.R. TAp73 acts via the bHLH Hey2 to promote long-term maintenance of neural precursors. Curr. Biol. 2010;20:2058–2065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

