Human Cytomegalovirus Promotes Survival of Infected Monocytes via a Distinct Temporal Regulation of Cellular Bcl-2 Family Proteins
- PMID: 26676786
- PMCID: PMC4810730
- DOI: 10.1128/JVI.01994-15
Human Cytomegalovirus Promotes Survival of Infected Monocytes via a Distinct Temporal Regulation of Cellular Bcl-2 Family Proteins
Abstract
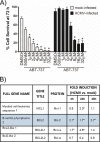
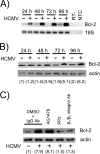
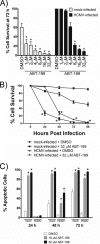
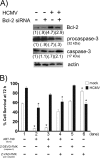
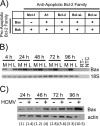
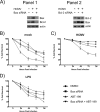
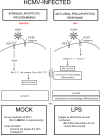
Monocytes play a key role in the hematogenous dissemination of human cytomegalovirus (HCMV) to target organ systems. To infect monocytes and reprogram them to deliver infectious virus, HCMV must overcome biological obstacles, including the short life span of monocytes and their antiviral proapoptotic response to infection. We have shown that virally induced upregulation of cellular Mcl-1 promotes early survival of HCMV-infected monocytes, allowing cells to overcome an early apoptotic checkpoint at around 48 h postinfection (hpi). Here, we demonstrate an HCMV-dependent shift from Mcl-1 as the primary antiapoptotic player to the related protein, Bcl-2, later during infection. Bcl-2 was upregulated in HCMV-infected monocytes beginning at 48 hpi. Treatment with the Bcl-2 antagonist ABT-199 only reduced the prosurvival effects of HCMV in target monocytes beginning at 48 hpi, suggesting that Mcl-1 controls survival prior to 48 hpi, while Bcl-2 promotes survival after 48 hpi. Although Bcl-2 was upregulated following viral binding/signaling through cellular integrins (compared to Mcl-1, which is upregulated through binding/activation of epidermal growth factor receptor [EGFR]), it functioned similarly to Mcl-1, adopting the early role of Mcl-1 in preventing caspase-3 cleavage/activation. This distinct, HCMV-induced shift from Mcl-1 to Bcl-2 occurs in response to a cellular upregulation of proapoptotic Bax, as small interfering RNA (siRNA)-mediated knockdown of Bax reduced the upregulation of Bcl-2 in infected monocytes and rescued the cells from the apoptotic effects of Bcl-2 inhibition. Our data demonstrate a distinct survival strategy whereby HCMV induces a biphasic regulation of cellular Bcl-2 proteins to promote host cell survival, leading to viral dissemination and the establishment of persistent HCMV infection.
Importance: Hematogenous dissemination of HCMV via infected monocytes is a crucial component of the viral survival strategy and is required for the establishment of persistent infection and for viral spread to additional hosts. Our system of infected primary human blood monocytes provides us with an opportunity to answer specific questions about viral spread and persistence in in vivo-relevant myeloid cells that cannot be addressed with the more traditionally used replication-permissive cells. Our goal in examining the mechanisms whereby HCMV reprograms infected monocytes to promote viral dissemination is to uncover new targets for therapeutic intervention that would disrupt key viral survival and persistence strategies. Because of this important role in maintaining survival of HCMV-infected monocytes, our new data on the role of Bcl-2 regulation during viral infection represents a promising molecular target for mitigating viral spread and persistence.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Human Cytomegalovirus Stimulates the Synthesis of Select Akt-Dependent Antiapoptotic Proteins during Viral Entry To Promote Survival of Infected Monocytes.J Virol. 2016 Jan 6;90(6):3138-47. doi: 10.1128/JVI.02879-15. J Virol. 2016. PMID: 26739047 Free PMC article.
-
PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes.J Immunol. 2010 Mar 15;184(6):3213-22. doi: 10.4049/jimmunol.0903025. Epub 2010 Feb 19. J Immunol. 2010. PMID: 20173022 Free PMC article.
-
Human Cytomegalovirus Induces an Atypical Activation of Akt To Stimulate the Survival of Short-Lived Monocytes.J Virol. 2016 Jun 24;90(14):6443-6452. doi: 10.1128/JVI.00214-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147739 Free PMC article.
-
HCMV reprogramming of infected monocyte survival and differentiation: a Goldilocks phenomenon.Viruses. 2014 Feb 13;6(2):782-807. doi: 10.3390/v6020782. Viruses. 2014. PMID: 24531335 Free PMC article. Review.
-
HCMV Infection and Apoptosis: How Do Monocytes Survive HCMV Infection?Viruses. 2018 Sep 29;10(10):533. doi: 10.3390/v10100533. Viruses. 2018. PMID: 30274264 Free PMC article. Review.
Cited by
-
Human Cytomegalovirus Manipulates Syntaxin 6 for Successful Trafficking and Subsequent Infection of Monocytes.J Virol. 2022 Jul 27;96(14):e0081922. doi: 10.1128/jvi.00819-22. Epub 2022 Jul 11. J Virol. 2022. PMID: 35862696 Free PMC article.
-
Viral binding-induced signaling drives a unique and extended intracellular trafficking pattern during infection of primary monocytes.Proc Natl Acad Sci U S A. 2016 Aug 2;113(31):8819-24. doi: 10.1073/pnas.1604317113. Epub 2016 Jul 18. Proc Natl Acad Sci U S A. 2016. PMID: 27432979 Free PMC article.
-
Human Cytomegalovirus UL135 Interacts with Host Adaptor Proteins To Regulate Epidermal Growth Factor Receptor and Reactivation from Latency.J Virol. 2018 Sep 26;92(20):e00919-18. doi: 10.1128/JVI.00919-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30089695 Free PMC article.
-
Molecular Determinants and the Regulation of Human Cytomegalovirus Latency and Reactivation.Viruses. 2018 Aug 20;10(8):444. doi: 10.3390/v10080444. Viruses. 2018. PMID: 30127257 Free PMC article. Review.
-
HCMV modulation of cellular PI3K/AKT/mTOR signaling: New opportunities for therapeutic intervention?Antiviral Res. 2019 Mar;163:82-90. doi: 10.1016/j.antiviral.2019.01.009. Epub 2019 Jan 19. Antiviral Res. 2019. PMID: 30668978 Free PMC article. Review.
References
-
- Mocarski E Jr, Shenk T, Griffiths PD, Pass R. 2013. Cytomegaloviruses, p 1960–2014. In Knipe DM, Howley PM (ed), Fields virology, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Nogalski MT, Collins-McMillen D, Yurochko AD. 2014. Overview of human cytomegalovirus pathogenesis, p 15–28. In Yurochko AD, Miller WE (ed), Human cytomegaloviruses: methods & protocols. Humana Press, New York, NY. - PubMed
-
- Cohen JI, Corey GR. 1985. Cytomegalovirus infection in the normal host. Medicine 64:100–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

