Estimating the probability of demonstrating vaccine efficacy in the declining Ebola epidemic: a Bayesian modelling approach
- PMID: 26671958
- PMCID: PMC4679933
- DOI: 10.1136/bmjopen-2015-009346
Estimating the probability of demonstrating vaccine efficacy in the declining Ebola epidemic: a Bayesian modelling approach
Abstract
Objectives: We investigate the chance of demonstrating Ebola vaccine efficacy in an individually randomised controlled trial implemented in the declining epidemic of Forécariah prefecture, Guinea.
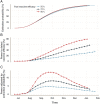
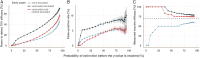
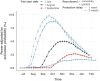
Methods: We extend a previously published dynamic transmission model to include a simulated individually randomised controlled trial of 100,000 participants. Using Bayesian methods, we fit the model to Ebola case incidence before a trial and forecast the expected dynamics until disease elimination. We simulate trials under these forecasts and test potential start dates and rollout schemes to assess power to detect efficacy, and bias in vaccine efficacy estimates that may be introduced.
Results: Under realistic assumptions, we found that a trial of 100,000 participants starting after 1 August had less than 5% chance of having enough cases to detect vaccine efficacy. In particular, gradual recruitment precludes detection of vaccine efficacy because the epidemic is likely to go extinct before enough participants are recruited. Exclusion of early cases in either arm of the trial creates bias in vaccine efficacy estimates.
Conclusions: The very low Ebola virus disease incidence in Forécariah prefecture means any individually randomised controlled trial implemented there is unlikely to be successful, unless there is a substantial increase in the number of cases.
Keywords: Clinical trials < THERAPEUTICS; Protocols & guidelines < HEALTH SERVICES ADMINISTRATION & MANAGEMENT; STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

Similar articles
-
Real-time dynamic modelling for the design of a cluster-randomized phase 3 Ebola vaccine trial in Sierra Leone.Vaccine. 2017 Jan 23;35(4):544-551. doi: 10.1016/j.vaccine.2016.12.019. Epub 2016 Dec 23. Vaccine. 2017. PMID: 28024952
-
Spatiotemporal dynamics of the Ebola epidemic in Guinea and implications for vaccination and disease elimination: a computational modeling analysis.BMC Med. 2016 Sep 7;14(1):130. doi: 10.1186/s12916-016-0678-3. BMC Med. 2016. PMID: 27600737 Free PMC article.
-
Attitudes about vaccines to prevent Ebola virus disease in Guinea at the end of a large Ebola epidemic: Results of a national household survey.Vaccine. 2017 Dec 14;35(49 Pt B):6915-6923. doi: 10.1016/j.vaccine.2017.06.026. Epub 2017 Jul 15. Vaccine. 2017. PMID: 28716555
-
Ebola vaccines in clinical trial: The promising candidates.Hum Vaccin Immunother. 2017 Jan 2;13(1):153-168. doi: 10.1080/21645515.2016.1225637. Epub 2016 Oct 20. Hum Vaccin Immunother. 2017. PMID: 27764560 Free PMC article. Review.
-
[Overview of the Ebola vaccines in pre-clinical and clinical development].Bull Soc Pathol Exot. 2016 Oct;109(4):256-261. doi: 10.1007/s13149-016-0521-2. Epub 2016 Sep 19. Bull Soc Pathol Exot. 2016. PMID: 27646961 Review. French.
Cited by
-
Quantifying Efficiency Gains of Innovative Designs of Two-Arm Vaccine Trials for COVID-19 Using an Epidemic Simulation Model.Stat Biopharm Res. 2022 Jan 2;14(1):33-41. doi: 10.1080/19466315.2021.1939774. Epub 2021 Jul 30. Stat Biopharm Res. 2022. PMID: 35096276 Free PMC article.
-
DESIGN OF VACCINE TRIALS DURING OUTBREAKS WITH AND WITHOUT A DELAYED VACCINATION COMPARATOR.Ann Appl Stat. 2018 Mar;12(1):330-347. doi: 10.1214/17-AOAS1095. Epub 2018 Mar 9. Ann Appl Stat. 2018. PMID: 29606991 Free PMC article.
-
Using simulation to aid trial design: Ring-vaccination trials.PLoS Negl Trop Dis. 2017 Mar 22;11(3):e0005470. doi: 10.1371/journal.pntd.0005470. eCollection 2017 Mar. PLoS Negl Trop Dis. 2017. PMID: 28328984 Free PMC article.
-
Outbreak analytics: a developing data science for informing the response to emerging pathogens.Philos Trans R Soc Lond B Biol Sci. 2019 Jul 8;374(1776):20180276. doi: 10.1098/rstb.2018.0276. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31104603 Free PMC article. Review.
-
Real-time forecasting of infectious disease dynamics with a stochastic semi-mechanistic model.Epidemics. 2018 Mar;22:56-61. doi: 10.1016/j.epidem.2016.11.003. Epub 2016 Dec 16. Epidemics. 2018. PMID: 28038870 Free PMC article.
References
-
- Centre for the Mathematical Modelling of Infectious Diseases. Visualisation and projections of the Ebola outbreak in West Africa. London Sch. Hyg. Trop. Med. http://ntncmch.github.io/ebola/ (accessed 7 Jun 2015).
-
- Kupferschmidt K. Scientists argue over access to remaining Ebola hotspots. Science. http://news.sciencemag.org/africa/2015/03/scientists-argue-over-access-r...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
