Histamine H2 Receptor-Mediated Suppression of Intestinal Inflammation by Probiotic Lactobacillus reuteri
- PMID: 26670383
- PMCID: PMC4701830
- DOI: 10.1128/mBio.01358-15
Histamine H2 Receptor-Mediated Suppression of Intestinal Inflammation by Probiotic Lactobacillus reuteri
Abstract
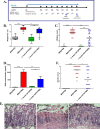
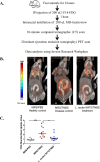
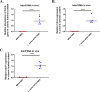
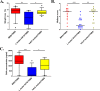
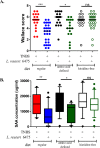
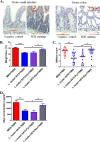
Probiotics and commensal intestinal microbes suppress mammalian cytokine production and intestinal inflammation in various experimental model systems. Limited information exists regarding potential mechanisms of probiotic-mediated immunomodulation in vivo. In this report, we demonstrate that specific probiotic strains of Lactobacillus reuteri suppress intestinal inflammation in a trinitrobenzene sulfonic acid (TNBS)-induced mouse colitis model. Only strains that possess the hdc gene cluster, including the histidine decarboxylase and histidine-histamine antiporter genes, can suppress colitis and mucosal cytokine (interleukin-6 [IL-6] and IL-1β in the colon) gene expression. Suppression of acute colitis in mice was documented by diminished weight loss, colonic injury, serum amyloid A (SAA) protein concentrations, and reduced uptake of [(18)F]fluorodeoxyglucose ([(18)F]FDG) in the colon by positron emission tomography (PET). The ability of probiotic L. reuteri to suppress colitis depends on the presence of a bacterial histidine decarboxylase gene(s) in the intestinal microbiome, consumption of a histidine-containing diet, and signaling via the histamine H2 receptor (H2R). Collectively, luminal conversion of l-histidine to histamine by hdc(+) L. reuteri activates H2R, and H2R signaling results in suppression of acute inflammation within the mouse colon.
Importance: Probiotics are microorganisms that when administered in adequate amounts confer beneficial effects on the host. Supplementation with probiotic strains was shown to suppress intestinal inflammation in patients with inflammatory bowel disease and in rodent colitis models. However, the mechanisms of probiosis are not clear. Our current studies suggest that supplementation with hdc(+) L. reuteri, which can convert l-histidine to histamine in the gut, resulted in suppression of colonic inflammation. These findings link luminal conversion of dietary components (amino acid metabolism) by gut microbes and probiotic-mediated suppression of colonic inflammation. The effective combination of diet, gut bacteria, and host receptor-mediated signaling may result in opportunities for therapeutic microbiology and provide clues for discovery and development of next-generation probiotics.
Copyright © 2015 Gao et al.
Figures

Comment in
-
IBD: Probiotics for IBD: a need for histamine?Nat Rev Gastroenterol Hepatol. 2016 Feb;13(2):62-3. doi: 10.1038/nrgastro.2016.2. Epub 2016 Jan 13. Nat Rev Gastroenterol Hepatol. 2016. PMID: 26758785 No abstract available.
Similar articles
-
Gut Microbe-Mediated Suppression of Inflammation-Associated Colon Carcinogenesis by Luminal Histamine Production.Am J Pathol. 2017 Oct;187(10):2323-2336. doi: 10.1016/j.ajpath.2017.06.011. Epub 2017 Sep 13. Am J Pathol. 2017. PMID: 28917668 Free PMC article.
-
Lactobacillus reuteri-specific immunoregulatory gene rsiR modulates histamine production and immunomodulation by Lactobacillus reuteri.J Bacteriol. 2013 Dec;195(24):5567-76. doi: 10.1128/JB.00261-13. Epub 2013 Oct 11. J Bacteriol. 2013. PMID: 24123819 Free PMC article.
-
FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri.Microbiologyopen. 2016 Oct;5(5):802-818. doi: 10.1002/mbo3.371. Epub 2016 Jun 28. Microbiologyopen. 2016. PMID: 27353144 Free PMC article.
-
Next-Generation Probiotic Therapy to Protect the Intestines From Injury.Front Cell Infect Microbiol. 2022 Jun 28;12:863949. doi: 10.3389/fcimb.2022.863949. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35837474 Free PMC article. Review.
-
Using murine colitis models to analyze probiotics-host interactions.FEMS Microbiol Rev. 2017 Aug 1;41(Supp_1):S49-S70. doi: 10.1093/femsre/fux035. FEMS Microbiol Rev. 2017. PMID: 28830096 Review.
Cited by
-
Benefits and concerns of probiotics: an overview of the potential genotoxicity of the colibactin-producing Escherichia coli Nissle 1917 strain.Gut Microbes. 2024 Jan-Dec;16(1):2397874. doi: 10.1080/19490976.2024.2397874. Epub 2024 Sep 4. Gut Microbes. 2024. PMID: 39229962 Free PMC article. Review.
-
Gut microbiota dysbiosis and its impact on asthma and other lung diseases: potential therapeutic approaches.Korean J Intern Med. 2024 Sep;39(5):746-758. doi: 10.3904/kjim.2023.451. Epub 2024 Aug 30. Korean J Intern Med. 2024. PMID: 39252487 Free PMC article. Review.
-
The Putative Antidepressant Mechanisms of Probiotic Bacteria: Relevant Genes and Proteins.Nutrients. 2021 May 10;13(5):1591. doi: 10.3390/nu13051591. Nutrients. 2021. PMID: 34068669 Free PMC article. Review.
-
Dysbiosis of the Urinary Microbiota Associated With Urine Levels of Proinflammatory Chemokine Interleukin-8 in Female Type 2 Diabetic Patients.Front Immunol. 2017 Aug 25;8:1032. doi: 10.3389/fimmu.2017.01032. eCollection 2017. Front Immunol. 2017. PMID: 28943876 Free PMC article.
-
Evaluation of Neuro-Hormonal Dynamics after the Administration of Probiotic Microbial Strains in a Murine Model of Hyperthyroidism.Nutrients. 2024 Apr 6;16(7):1077. doi: 10.3390/nu16071077. Nutrients. 2024. PMID: 38613110 Free PMC article.
References
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. 2012. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142:46–54. doi:10.1053/j.gastro.2011.10.001. - DOI - PubMed
-
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105:16731–16736. doi:10.1073/pnas.0804812105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
