BACE-1 is expressed in the blood-brain barrier endothelium and is upregulated in a murine model of Alzheimer's disease
- PMID: 26661166
- PMCID: PMC4929696
- DOI: 10.1177/0271678X15606463
BACE-1 is expressed in the blood-brain barrier endothelium and is upregulated in a murine model of Alzheimer's disease
Abstract
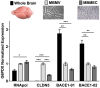
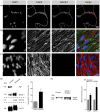
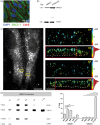
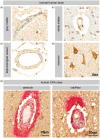
Endothelial cells of the blood-brain barrier form a structural and functional barrier maintaining brain homeostasis via paracellular tight junctions and specific transporters such as P-glycoprotein. The blood-brain barrier is responsible for negligible bioavailability of many neuroprotective drugs. In Alzheimer's disease, current treatment approaches include inhibitors of BACE-1 (β-site of amyloid precursor protein cleaving enzyme), a proteinase generating neurotoxic β-amyloid. It is known that BACE-1 is highly expressed in endosomes and membranes of neurons and glia. We now provide evidence that BACE-1 is expressed in blood-brain barrier endothelial cells of human, mouse, and bovine origin. We further show its predominant membrane localization by 3D-dSTORM super-resolution microscopy, and by biochemical fractionation that further shows an abluminal distribution of BACE-1 in brain microvessels. We confirm its functionality in processing APP in primary mouse brain endothelial cells. In an Alzheimer's disease mouse model we show that BACE-1 is upregulated at the blood-brain barrier compared to healthy controls. We therefore suggest a critical role for BACE-1 at the blood-brain barrier in β-amyloid generation and in vascular aspects of Alzheimer's disease, particularly in the development of cerebral amyloid angiopathy.
Keywords: Alzheimer’s disease; BACE-1; blood–brain barrier; endothelium; β-amyloid.
© The Author(s) 2015.
Figures

Similar articles
-
MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer's disease.Int J Mol Med. 2014 Jul;34(1):160-6. doi: 10.3892/ijmm.2014.1780. Epub 2014 May 13. Int J Mol Med. 2014. PMID: 24827165
-
Deposition of BACE-1 Protein in the Brains of APP/PS1 Double Transgenic Mice.Biomed Res Int. 2016;2016:8380618. doi: 10.1155/2016/8380618. Epub 2016 May 18. Biomed Res Int. 2016. PMID: 27294139 Free PMC article.
-
Neuronal and glial beta-secretase (BACE) protein expression in transgenic Tg2576 mice with amyloid plaque pathology.J Neurosci Res. 2001 Jun 1;64(5):437-46. doi: 10.1002/jnr.1095. J Neurosci Res. 2001. PMID: 11391698
-
Functions of the Alzheimer's Disease Protease BACE1 at the Synapse in the Central Nervous System.J Mol Neurosci. 2016 Nov;60(3):305-315. doi: 10.1007/s12031-016-0800-1. Epub 2016 Jul 25. J Mol Neurosci. 2016. PMID: 27456313 Free PMC article. Review.
-
BACE: Therapeutic target and potential biomarker for Alzheimer's disease.Int J Biochem Cell Biol. 2010 Dec;42(12):1923-6. doi: 10.1016/j.biocel.2010.08.017. Epub 2010 Sep 15. Int J Biochem Cell Biol. 2010. PMID: 20817005 Review.
Cited by
-
VasoTracker, a Low-Cost and Open Source Pressure Myograph System for Vascular Physiology.Front Physiol. 2019 Feb 21;10:99. doi: 10.3389/fphys.2019.00099. eCollection 2019. Front Physiol. 2019. PMID: 30846942 Free PMC article.
-
Expression and Processing of Amyloid Precursor Protein in Vascular Endothelium.Physiology (Bethesda). 2017 Jan;32(1):20-32. doi: 10.1152/physiol.00021.2016. Physiology (Bethesda). 2017. PMID: 27927802 Free PMC article. Review.
-
Sortilin Fragments Deposit at Senile Plaques in Human Cerebrum.Front Neuroanat. 2017 Jun 7;11:45. doi: 10.3389/fnana.2017.00045. eCollection 2017. Front Neuroanat. 2017. PMID: 28638323 Free PMC article.
-
The Blood-Brain Barrier in Alzheimer's Disease.Handb Exp Pharmacol. 2022;273:247-266. doi: 10.1007/164_2020_418. Handb Exp Pharmacol. 2022. PMID: 33580390 Free PMC article.
-
Biomimetic Nanocarrier Targeting Drug(s) to Upstream-Receptor Mechanisms in Dementia: Focusing on Linking Pathogenic Cascades.Biomimetics (Basel). 2020 Mar 20;5(1):11. doi: 10.3390/biomimetics5010011. Biomimetics (Basel). 2020. PMID: 32244941 Free PMC article. Review.
References
-
- Probst G, Xu Y-Z. Small-molecule BACE1 inhibitors: a patent literature review (2006-2011). Expert Opin Ther Pat 2012; 22: 511–540. - PubMed
-
- Bush AI, Martins RN, Rumble B, et al. The amyloid precursor protein of Alzheimer's disease is released by human platelets. J Biol Chem 1990; 265: 15977–15983. - PubMed
-
- Bush AI, Beyreuther K, Masters CL. The beta A4 amyloid protein precursor in human circulation. Ann N Y Acad Sci 1993; 695: 175–182. - PubMed
-
- Ho L, Fukuchi KI, Younkin SG. The alternatively spliced Kunitz protease inhibitor domain alters amyloid beta protein precursor processing and amyloid beta protein production in cultured cells. J Biol Chem 1996; 271: 30929–30934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

