Early apoptosis of porcine alveolar macrophages limits avian influenza virus replication and pro-inflammatory dysregulation
- PMID: 26642934
- PMCID: PMC4672291
- DOI: 10.1038/srep17999
Early apoptosis of porcine alveolar macrophages limits avian influenza virus replication and pro-inflammatory dysregulation
Abstract
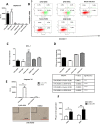
Pigs are evidently more resistant to avian than swine influenza A viruses, mediated in part through frontline epithelial cells and alveolar macrophages (AM). Although porcine AM (PAM) are crucial in influenza virus control, their mode of control is unclear. To gain insight into the possible role of PAM in the mediation of avian influenza virus resistance, we compared the host effects and replication of two avian (H2N3 and H6N1) and three mammalian (swine H1N1, human H1N1 and pandemic H1N1) influenza viruses in PAM. We found that PAM were readily susceptible to initial infection with all five avian and mammalian influenza viruses but only avian viruses caused early and extensive apoptosis (by 6 h of infection) resulting in reduced virus progeny and moderated pro-inflammation. Full length viral PB1-F2 present only in avian influenza viruses is a virulence factor that targets AM for mitochondrial-associated apoptotic cell death. With the use of reverse genetics on an avian H5N1 virus, we found that full length PB1-F2 contributed to increased apoptosis and pro-inflammation but not to reduced virus replication. Taken together, we propose that early apoptosis of PAM limits the spread of avian influenza viruses and that PB1-F2 could play a contributory role in the process.
Figures


Similar articles
-
Emergence of Highly Pathogenic Avian Influenza A(H5N1) Virus PB1-F2 Variants and Their Virulence in BALB/c Mice.J Virol. 2015 Jun;89(11):5835-46. doi: 10.1128/JVI.03137-14. Epub 2015 Mar 18. J Virol. 2015. PMID: 25787281 Free PMC article.
-
Strain-dependent effects of PB1-F2 of triple-reassortant H3N2 influenza viruses in swine.J Gen Virol. 2012 Oct;93(Pt 10):2204-2214. doi: 10.1099/vir.0.045005-0. Epub 2012 Jul 18. J Gen Virol. 2012. PMID: 22815274 Free PMC article.
-
Swine alveolar macrophage cell model allows optimal replication of influenza A viruses regardless of their origin.Virology. 2016 Mar;490:91-8. doi: 10.1016/j.virol.2016.01.006. Epub 2016 Feb 6. Virology. 2016. PMID: 26855331
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
-
Isolation and genetic characterization of avian-like H1N1 and novel ressortant H1N2 influenza viruses from pigs in China.Biochem Biophys Res Commun. 2009 Aug 21;386(2):278-83. doi: 10.1016/j.bbrc.2009.05.056. Epub 2009 May 19. Biochem Biophys Res Commun. 2009. PMID: 19460353 Review.
Cited by
-
Amino acid 138 in the HA of a H3N2 subtype influenza A virus increases affinity for the lower respiratory tract and alveolar macrophages in pigs.PLoS Pathog. 2024 Feb 20;20(2):e1012026. doi: 10.1371/journal.ppat.1012026. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38377132 Free PMC article.
-
Comparative transcriptomics reveals small RNA composition and differential microRNA responses underlying interferon-mediated antiviral regulation in porcine alveolar macrophages.Front Immunol. 2022 Oct 28;13:1016268. doi: 10.3389/fimmu.2022.1016268. eCollection 2022. Front Immunol. 2022. PMID: 36389683 Free PMC article.
-
Influenza Virus Overcomes Cellular Blocks To Productively Replicate, Impacting Macrophage Function.J Virol. 2017 Jan 3;91(2):e01417-16. doi: 10.1128/JVI.01417-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807237 Free PMC article.
-
Influenza viral matrix 1 protein aggravates viral pathogenicity by inducing TLR4-mediated reactive oxygen species production and apoptotic cell death.Cell Death Dis. 2023 Mar 30;14(3):228. doi: 10.1038/s41419-023-05749-5. Cell Death Dis. 2023. PMID: 36990977 Free PMC article.
-
Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus.Emerg Infect Dis. 2024 Jul;30(7):1361-1373. doi: 10.3201/eid3007.240689. Epub 2024 Jun 11. Emerg Infect Dis. 2024. PMID: 38861554 Free PMC article.
References
-
- Olsen C. W., Brown I. H., Easterday B. C. & Van Reeth K. Swine influenza. in Diseases of swine (eds. Straw B. E., Zimmerman J. J., D'Allaire S. & Taylor D. J. ) 469–482 (Blackwell Publishing, Oxford, 2006).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

