Oncolysis by paramyxoviruses: multiple mechanisms contribute to therapeutic efficiency
- PMID: 26640816
- PMCID: PMC4667958
- DOI: 10.1038/mto.2015.11
Oncolysis by paramyxoviruses: multiple mechanisms contribute to therapeutic efficiency
Abstract
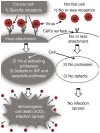
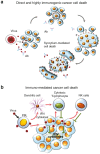
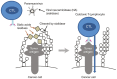
Oncolytic paramyxoviruses include some strains of Measles, Mumps, Newcastle disease, and Sendai viruses. All these viruses are well equipped for promoting highly specific and efficient malignant cell death, which can be direct and/or immuno-mediated. A number of proteins that serve as natural receptors for oncolytic paramyxoviruses are frequently overexpressed in malignant cells. Therefore, the preferential interaction of paramyxoviruses with malignant cells rather than with normal cells is promoted. Due to specific genetic defects of cancer cells in the interferon (IFN) and apoptotic pathways, viral replication has the potential to be promoted specifically in tumors. Viral mediation of syncytium formation (a polykaryonic structure) promotes intratumoral paramyxo-virus replication and spreading, without exposure to host neutralizing antibodies. So, two related processes: efficient intratumoral infection spread as well as the consequent mass malignant cell death, both are enhanced. In general, the paramyxoviruses elicit strong anticancer innate and adaptive immune responses by triggering multiple danger signals. The paramyxoviruses are powerful inducers of IFN and other immuno-stimulating cytokines. These viruses efficiently promote anticancer activity of natural killer cells, dendritic cells, and cytotoxic T lymphocytes. Moreover, a neuraminidase (sialidase), a component of the viral envelope of Newcastle Disease, Mumps, and Sendai viruses, can cleave sialic acids on the surface of malignant cells thereby unmasking cancer antigens and exposing them to the immune system. These multiple mechanisms contribute to therapeutic efficacy of oncolytic paramyxovi-ruses and are responsible for encouraging results in preclinical and clinical studies.
Conflict of interest statement
Olga V. Matveeva is a co-author of a patent entitled “Method for cancer immunotherapy and pharmaceutical compositions based on oncolytic non-pathogenic Sendai virus.”
Figures
Similar articles
-
Prospects for Using Expression Patterns of Paramyxovirus Receptors as Biomarkers for Oncolytic Virotherapy.Cancers (Basel). 2020 Dec 5;12(12):3659. doi: 10.3390/cancers12123659. Cancers (Basel). 2020. PMID: 33291506 Free PMC article. Review.
-
Mechanisms of Oncolysis by Paramyxovirus Sendai.Acta Naturae. 2015 Apr-Jun;7(2):6-16. Acta Naturae. 2015. PMID: 26085940 Free PMC article.
-
[Oncolytic Paramyxoviruses: Mechanism of Action, Preclinical and Clinical Studies].Mol Biol (Mosk). 2018 May-Jun;52(3):360-379. doi: 10.7868/S0026898418030023. Mol Biol (Mosk). 2018. PMID: 29989571 Review. Russian.
-
Oncolysis by paramyxoviruses: preclinical and clinical studies.Mol Ther Oncolytics. 2015;2:15017-. doi: 10.1038/mto.2015.17. Epub 2015 Oct 21. Mol Ther Oncolytics. 2015. PMID: 26640815 Free PMC article.
-
Newcastle Disease Virus Establishes Persistent Infection in Tumor Cells In Vitro: Contribution of the Cleavage Site of Fusion Protein and Second Sialic Acid Binding Site of Hemagglutinin-Neuraminidase.J Virol. 2017 Jul 27;91(16):e00770-17. doi: 10.1128/JVI.00770-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28592535 Free PMC article.
Cited by
-
Directed evolution as a tool for the selection of oncolytic RNA viruses with desired phenotypes.Oncolytic Virother. 2019 Jul 12;8:9-26. doi: 10.2147/OV.S176523. eCollection 2019. Oncolytic Virother. 2019. PMID: 31372363 Free PMC article.
-
Prospects for Using Expression Patterns of Paramyxovirus Receptors as Biomarkers for Oncolytic Virotherapy.Cancers (Basel). 2020 Dec 5;12(12):3659. doi: 10.3390/cancers12123659. Cancers (Basel). 2020. PMID: 33291506 Free PMC article. Review.
-
Anti-cancer Virotherapy in Russia: Lessons from the Past, Current Challenges and Prospects for the Future.Curr Pharm Biotechnol. 2023;24(2):266-278. doi: 10.2174/1389201023666220516121813. Curr Pharm Biotechnol. 2023. PMID: 35578840 Review.
-
Optimization of oncolytic effect of Newcastle disease virus Clone30 by selecting sensitive tumor host and constructing more oncolytic viruses.Gene Ther. 2021 Dec;28(12):697-717. doi: 10.1038/s41434-020-0145-9. Epub 2020 May 14. Gene Ther. 2021. Retraction in: Gene Ther. 2024 Jul;31(7-8):436. doi: 10.1038/s41434-024-00459-9 PMID: 32409746 Free PMC article. Retracted.
-
Complete Genome Sequence of the Oncolytic Sendai virus Strain Moscow.Genome Announc. 2016 Aug 11;4(4):e00818-16. doi: 10.1128/genomeA.00818-16. Genome Announc. 2016. PMID: 27516510 Free PMC article.
References
-
- Lamb, RA and Parks, GD (2007). Paramyxoviridae: the viruses and their replication. In: Knipe, DM and Howley PM (eds). Fields Virology, 5th edn. Lippincott Williams & Wilkins: Philadelphia. pp. 1449–1496.
-
- Villar, E and Barroso, IM (2006). Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj J 23: 5–17. - PubMed
-
- Cantín, C, Holguera, J, Ferreira, L, Villar, E and Muñoz-Barroso, I (2007). Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J Gen Virol 88(Pt 2): 559–569. - PubMed
-
- Higuchi, H, Bronk, SF, Bateman, A, Harrington, K, Vile, RG and Gores, GJ (2000). Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res 60: 6396–6402. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

