Collecting Fecal Samples for Microbiome Analyses in Epidemiology Studies
- PMID: 26604270
- PMCID: PMC4821594
- DOI: 10.1158/1055-9965.EPI-15-0951
Collecting Fecal Samples for Microbiome Analyses in Epidemiology Studies
Abstract
Background: The need to develop valid methods for sampling and analyzing fecal specimens for microbiome studies is increasingly important, especially for large population studies.
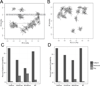
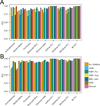
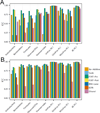
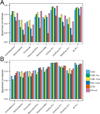
Methods: Some of the most important attributes of any sampling method are reproducibility, stability, and accuracy. We compared seven fecal sampling methods [no additive, RNAlater, 70% ethanol, EDTA, dry swab, and pre/post development fecal occult blood test (FOBT)] using 16S rRNA microbiome profiling in two laboratories. We evaluated nine commonly used microbiome metrics: abundance of three phyla, two alpha-diversities, and four beta-diversities. We determined the technical reproducibility, stability at ambient temperature, and accuracy.
Results: Although microbiome profiles showed systematic biases according to sample method and time at ambient temperature, the highest source of variation was between individuals. All collection methods showed high reproducibility. FOBT and RNAlater resulted in the highest stability without freezing for 4 days. In comparison with no-additive samples, swab, FOBT, and 70% ethanol exhibited the greatest accuracy when immediately frozen.
Conclusions: Overall, optimal stability and reproducibility were achieved using FOBT, making this a reasonable sample collection method for 16S analysis.
Impact: Having standardized method of collecting and storing stable fecal samples will allow future investigations into the role of gut microbiota in chronic disease etiology in large population studies.
©2015 American Association for Cancer Research.
Figures


Comment in
-
Fecal Microbiome in Epidemiologic Studies-Letter.Cancer Epidemiol Biomarkers Prev. 2016 May;25(5):869. doi: 10.1158/1055-9965.EPI-16-0063. Epub 2016 Mar 9. Cancer Epidemiol Biomarkers Prev. 2016. PMID: 26961995 Free PMC article. No abstract available.
Similar articles
-
Comparison of Fecal Collection Methods for Microbiota Studies in Bangladesh.Appl Environ Microbiol. 2017 May 1;83(10):e00361-17. doi: 10.1128/AEM.00361-17. Print 2017 May 15. Appl Environ Microbiol. 2017. PMID: 28258145 Free PMC article.
-
Comparison of Methods To Collect Fecal Samples for Microbiome Studies Using Whole-Genome Shotgun Metagenomic Sequencing.mSphere. 2020 Feb 26;5(1):e00827-19. doi: 10.1128/mSphere.00827-19. mSphere. 2020. PMID: 32250964 Free PMC article.
-
Comparison of Fecal Collection Methods on Variation in Gut Metagenomics and Untargeted Metabolomics.mSphere. 2021 Oct 27;6(5):e0063621. doi: 10.1128/mSphere.00636-21. Epub 2021 Sep 15. mSphere. 2021. PMID: 34523982 Free PMC article.
-
Comparison of fecal and oral collection methods for studies of the human microbiota in two Iranian cohorts.BMC Microbiol. 2021 Nov 22;21(1):324. doi: 10.1186/s12866-021-02387-9. BMC Microbiol. 2021. PMID: 34809575 Free PMC article.
-
Comparison of Collection Methods for Fecal Samples in Microbiome Studies.Am J Epidemiol. 2017 Jan 15;185(2):115-123. doi: 10.1093/aje/kww177. Epub 2016 Dec 16. Am J Epidemiol. 2017. PMID: 27986704 Free PMC article.
Cited by
-
Using fecal immmunochemical cartridges for gut microbiome analysis within a colorectal cancer screening program.Gut Microbes. 2023 Jan-Dec;15(1):2176119. doi: 10.1080/19490976.2023.2176119. Gut Microbes. 2023. PMID: 36794815 Free PMC article.
-
Impact of Sample Preservation and Manipulation on Insect Gut Microbiome Profiling. A Test Case With Fruit Flies (Diptera, Tephritidae).Front Microbiol. 2019 Dec 13;10:2833. doi: 10.3389/fmicb.2019.02833. eCollection 2019. Front Microbiol. 2019. PMID: 31921020 Free PMC article.
-
Assessment of the impact of different fecal storage protocols on the microbiota diversity and composition: a pilot study.BMC Microbiol. 2019 Jun 28;19(1):145. doi: 10.1186/s12866-019-1519-2. BMC Microbiol. 2019. PMID: 31253096 Free PMC article.
-
Variations of Gut Microbiome Profile Under Different Storage Conditions and Preservation Periods: A Multi-Dimensional Evaluation.Front Microbiol. 2020 May 27;11:972. doi: 10.3389/fmicb.2020.00972. eCollection 2020. Front Microbiol. 2020. PMID: 32536906 Free PMC article.
-
Evaluation of Sample Preservation and Storage Methods for Metaproteomics Analysis of Intestinal Microbiomes.Microbiol Spectr. 2021 Dec 22;9(3):e0187721. doi: 10.1128/Spectrum.01877-21. Epub 2021 Dec 15. Microbiol Spectr. 2021. PMID: 34908431 Free PMC article.
References
-
- Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–875. e1–e3. - PubMed
-
- Estrada-Velasco BI, Cruz M, Garcia-Mena J, Valladares Salgado A, Peralta Romero J, Guna Serrano Mde L, et al. Childhood obesity is associated to the interaction between firmicutes and high energy food consumption. Nutr Hosp. 2014;31:1074–1081. - PubMed
-
- Engsbro AL, Stensvold CR, Vedel Nielsen H, Bytzer P. Prevalence, incidence, and risk factors of intestinal parasites in Danish primary care patients with irritable bowel syndrome. Scand J Infect Dis. 2014;46:204–209. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

