Myofibroblastic Conversion and Regeneration of Mesothelial Cells in Peritoneal and Liver Fibrosis
- PMID: 26598235
- PMCID: PMC4729239
- DOI: 10.1016/j.ajpath.2015.08.009
Myofibroblastic Conversion and Regeneration of Mesothelial Cells in Peritoneal and Liver Fibrosis
Abstract
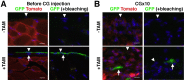
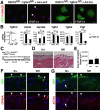
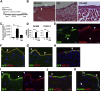
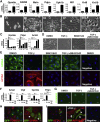
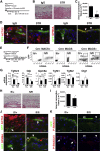
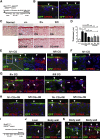
Mesothelial cells (MCs) form a single epithelial layer and line the surface of body cavities and internal organs. Patients who undergo peritoneal dialysis often develop peritoneal fibrosis that is characterized by the accumulation of myofibroblasts in connective tissue. Although MCs are believed to be the source of myofibroblasts, their contribution has remained obscure. We determined the contribution of peritoneal MCs to myofibroblasts in chlorhexidine gluconate (CG)-induced fibrosis compared with that of phenotypic changes of liver MCs. CG injections resulted in disappearance of MCs from the body wall and the accumulation of myofibroblasts in the connective tissue. Conditional linage tracing with Wilms tumor 1 (Wt1)-CreERT2 and Rosa26 reporter mice found that 17% of myofibroblasts were derived from MCs in peritoneal fibrosis. Conditional deletion of transforming growth factor-β type II receptor in Wt1(+) MCs substantially reduced peritoneal fibrosis. The CG treatment also induced myofibroblastic conversion of MCs in the liver. Lineage tracing with Mesp1-Cre mice revealed that Mesp1(+) mesoderm gave rise to liver MCs but not peritoneal MCs. During recovery from peritoneal fibrosis, peritoneal MCs, but not liver MCs, contribute to the regeneration of the peritoneal mesothelium, indicating an inherent difference between parietal and visceral MCs. In conclusion, MCs partially contribute to myofibroblasts in peritoneal and liver fibrosis, and protection of the MC layer leads to reduced development of fibrous tissue.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The Role of Mesothelial Cells in Liver Development, Injury, and Regeneration.Gut Liver. 2016 Mar;10(2):166-76. doi: 10.5009/gnl15226. Gut Liver. 2016. PMID: 26934883 Free PMC article. Review.
-
Role of TGF-β signaling in differentiation of mesothelial cells to vitamin A-poor hepatic stellate cells in liver fibrosis.Am J Physiol Gastrointest Liver Physiol. 2016 Feb 15;310(4):G262-72. doi: 10.1152/ajpgi.00257.2015. Epub 2015 Dec 23. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26702136 Free PMC article.
-
Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury.Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2324-9. doi: 10.1073/pnas.1214136110. Epub 2013 Jan 23. Proc Natl Acad Sci U S A. 2013. PMID: 23345421 Free PMC article.
-
Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis.Sci Rep. 2017 Jul 14;7(1):5392. doi: 10.1038/s41598-017-05624-2. Sci Rep. 2017. PMID: 28710437 Free PMC article.
-
Transition of mesothelial cell to fibroblast in peritoneal dialysis: EMT, stem cell or bystander?Perit Dial Int. 2015 Jan-Feb;35(1):14-25. doi: 10.3747/pdi.2014.00188. Perit Dial Int. 2015. PMID: 25700459 Free PMC article. Review.
Cited by
-
Fibroblast Heterogeneity in and Its Implications for Plastic and Reconstructive Surgery: A Basic Science Review.Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2927. doi: 10.1097/GOX.0000000000002927. eCollection 2020 Jun. Plast Reconstr Surg Glob Open. 2020. PMID: 32766071 Free PMC article.
-
The Role of Mesothelial Cells in Liver Development, Injury, and Regeneration.Gut Liver. 2016 Mar;10(2):166-76. doi: 10.5009/gnl15226. Gut Liver. 2016. PMID: 26934883 Free PMC article. Review.
-
Mechanisms of liver fibrosis and its role in liver cancer.Exp Biol Med (Maywood). 2020 Jan;245(2):96-108. doi: 10.1177/1535370219898141. Epub 2020 Jan 10. Exp Biol Med (Maywood). 2020. PMID: 31924111 Free PMC article. Review.
-
Vascular disease persistence in giant cell arteritis: are stromal cells neglected?Ann Rheum Dis. 2024 Aug 27;83(9):1100-1109. doi: 10.1136/ard-2023-225270. Ann Rheum Dis. 2024. PMID: 38684323 Free PMC article. Review.
-
Histone deacetylase 6 inhibition counteracts the epithelial-mesenchymal transition of peritoneal mesothelial cells and prevents peritoneal fibrosis.Oncotarget. 2017 Sep 18;8(51):88730-88750. doi: 10.18632/oncotarget.20982. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179471 Free PMC article.
References
-
- Mutsaers S.E. The mesothelial cell. Int J Biochem Cell Biol. 2004;36:9–16. - PubMed
-
- Saga Y., Miyagawa-Tomita S., Takagi A., Kitajima S., Miyazaki Ji, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

