Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort
- PMID: 26581300
- PMCID: PMC4691687
- DOI: 10.1212/WNL.0000000000002220
Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort
Abstract
Objective: To assess the genetic contribution of TBK1, a gene implicated in amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and FTD-ALS, in Belgian FTD and ALS patient cohorts containing a significant part of genetically unresolved patients.
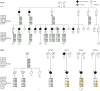
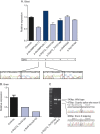
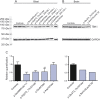
Methods: We sequenced TBK1 in a hospital-based cohort of 482 unrelated patients with FTD and FTD-ALS and 147 patients with ALS and an extended Belgian FTD-ALS family DR158. We followed up mutation carriers by segregation studies, transcript and protein expression analysis, and immunohistochemistry.
Results: We identified 11 patients carrying a loss-of-function (LOF) mutation resulting in an overall mutation frequency of 1.7% (11/629), 1.1% in patients with FTD (5/460), 3.4% in patients with ALS (5/147), and 4.5% in patients with FTD-ALS (1/22). We found 1 LOF mutation, p.Glu643del, in 6 unrelated patients segregating with disease in family DR158. Of 2 mutation carriers, brain and spinal cord was characterized by TDP-43-positive pathology. The LOF mutations including the p.Glu643del mutation led to loss of transcript or protein in blood and brain.
Conclusions: TBK1 LOF mutations are the third most frequent cause of clinical FTD in the Belgian clinically based patient cohort, after C9orf72 and GRN, and the second most common cause of clinical ALS after C9orf72. These findings reinforce that FTD and ALS belong to the same disease continuum.
© 2015 American Academy of Neurology.
Figures

Similar articles
-
Clinical features of TBK1 carriers compared with C9orf72, GRN and non-mutation carriers in a Belgian cohort.Brain. 2016 Feb;139(Pt 2):452-67. doi: 10.1093/brain/awv358. Epub 2015 Dec 15. Brain. 2016. PMID: 26674655 Free PMC article.
-
TBK1 mutation frequencies in French frontotemporal dementia and amyotrophic lateral sclerosis cohorts.Neurobiol Aging. 2015 Nov;36(11):3116.e5-3116.e8. doi: 10.1016/j.neurobiolaging.2015.08.009. Epub 2015 Aug 14. Neurobiol Aging. 2015. PMID: 26476236
-
Association between TBK1 mutations and risk of amyotrophic lateral sclerosis/frontotemporal dementia spectrum: a meta-analysis.Neurol Sci. 2018 May;39(5):811-820. doi: 10.1007/s10072-018-3246-0. Epub 2018 Jan 18. Neurol Sci. 2018. PMID: 29349657 Review.
-
TBK1 Mutation Spectrum in an Extended European Patient Cohort with Frontotemporal Dementia and Amyotrophic Lateral Sclerosis.Hum Mutat. 2017 Mar;38(3):297-309. doi: 10.1002/humu.23161. Epub 2017 Jan 19. Hum Mutat. 2017. PMID: 28008748 Free PMC article.
-
Sex differences in the prevalence of genetic mutations in FTD and ALS: A meta-analysis.Neurology. 2017 Oct 10;89(15):1633-1642. doi: 10.1212/WNL.0000000000004494. Epub 2017 Sep 15. Neurology. 2017. PMID: 28916533 Free PMC article. Review.
Cited by
-
What we know about TMEM106B in neurodegeneration.Acta Neuropathol. 2016 Nov;132(5):639-651. doi: 10.1007/s00401-016-1610-9. Epub 2016 Aug 20. Acta Neuropathol. 2016. PMID: 27543298 Free PMC article. Review.
-
Rare TBK1 variants in patients with frontotemporal dementia and amyotrophic lateral sclerosis in a Chinese cohort.Transl Neurodegener. 2018 Dec 4;7:31. doi: 10.1186/s40035-018-0136-6. eCollection 2018. Transl Neurodegener. 2018. PMID: 30534373 Free PMC article.
-
Causative Genes in Amyotrophic Lateral Sclerosis and Protein Degradation Pathways: a Link to Neurodegeneration.Mol Neurobiol. 2018 Aug;55(8):6480-6499. doi: 10.1007/s12035-017-0856-0. Epub 2018 Jan 10. Mol Neurobiol. 2018. PMID: 29322304 Review.
-
Genetic and clinical landscape of Chinese frontotemporal dementia: dominance of TBK1 and OPTN mutations.Alzheimers Res Ther. 2024 Jun 13;16(1):127. doi: 10.1186/s13195-024-01493-w. Alzheimers Res Ther. 2024. PMID: 38872230 Free PMC article.
-
Network analysis of the progranulin-deficient mouse brain proteome reveals pathogenic mechanisms shared in human frontotemporal dementia caused by GRN mutations.Acta Neuropathol Commun. 2020 Oct 7;8(1):163. doi: 10.1186/s40478-020-01037-x. Acta Neuropathol Commun. 2020. PMID: 33028409 Free PMC article.
References
-
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 2002;59:1077–1079. - PubMed
-
- Tsuji H, Arai T, Kametani F, et al. Molecular analysis and biochemical classification of TDP-43 proteinopathy. Brain 2012;135:3380–3391. - PubMed
-
- Gijselinck I, Van Langenhove T, van der Zee J, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 2012;11:54–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
