Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells
- PMID: 26577569
- PMCID: PMC4871788
- DOI: 10.1038/mi.2015.118
Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells
Abstract
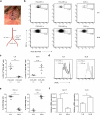
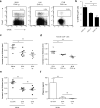
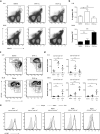
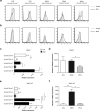
Tolerance to harmless exogenous antigens is the default immune response in the gastrointestinal tract. Although extensive studies have demonstrated the importance of the mesenteric lymph nodes (MLNs) and intestinal CD103(+) dendritic cells (DCs) in driving small intestinal tolerance to protein antigen, the structural and immunological basis of colonic tolerance remain poorly understood. We show here that the caudal and iliac lymph nodes (ILNs) are inductive sites for distal colonic immune responses and that colonic T cell-mediated tolerance induction to protein antigen is initiated in these draining lymph nodes and not in MLNs. In agreement, colonic tolerance induction was not altered by mesenteric lymphadenectomy. Despite tolerance development, CD103(+)CD11b(+) DCs, which are the major migratory DC population in the MLNs, and the tolerance-related retinoic acid-generating enzyme RALDH2 were virtually absent from the ILNs. Administration of ovalbumin (OVA) to the distal colon did increase the number of CD11c(+)MHCII(hi) migratory CD103(-)CD11b(+) and CD103(+)CD11b(-) DCs in the ILNs. Strikingly, colonic tolerance was intact in Batf3-deficient mice specifically lacking CD103(+)CD11b(-) DCs, suggesting that CD103(-) DCs in the ILNs are sufficient to drive tolerance induction after protein antigen encounter in the distal colon. Altogether, we identify different inductive sites for small intestinal and colonic T-cell responses and reveal that distinct cellular mechanisms are operative to maintain tolerance at these sites.
Figures

Similar articles
-
Mesenteric lymph node CD11b- CD103+ PD-L1High dendritic cells highly induce regulatory T cells.Immunology. 2017 Sep;152(1):52-64. doi: 10.1111/imm.12747. Epub 2017 Jun 1. Immunology. 2017. PMID: 28423181 Free PMC article.
-
Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells.J Exp Med. 2010 Apr 12;207(4):823-36. doi: 10.1084/jem.20091627. Epub 2010 Mar 29. J Exp Med. 2010. PMID: 20351058 Free PMC article.
-
A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity.J Immunol. 2011 Jun 1;186(11):6287-95. doi: 10.4049/jimmunol.1004036. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525388
-
Subsets of migrating intestinal dendritic cells.Immunol Rev. 2010 Mar;234(1):259-67. doi: 10.1111/j.0105-2896.2009.00866.x. Immunol Rev. 2010. PMID: 20193024 Review.
-
Intestinal CD103+ dendritic cells: master regulators of tolerance?Trends Immunol. 2011 Sep;32(9):412-9. doi: 10.1016/j.it.2011.06.003. Epub 2011 Aug 2. Trends Immunol. 2011. PMID: 21816673 Review.
Cited by
-
IL-10 signaling in dendritic cells controls IL-1β-mediated IFNγ secretion by human CD4+ T cells: relevance to inflammatory bowel disease.Mucosal Immunol. 2019 Sep;12(5):1201-1211. doi: 10.1038/s41385-019-0194-9. Epub 2019 Aug 15. Mucosal Immunol. 2019. PMID: 31417161 Free PMC article.
-
Single-Cell Protein and RNA Expression Analysis of Mononuclear Phagocytes in Intestinal Mucosa and Mesenteric Lymph Nodes of Ulcerative Colitis and Crohn's Disease Patients.Cells. 2020 Mar 27;9(4):813. doi: 10.3390/cells9040813. Cells. 2020. PMID: 32230977 Free PMC article. Review.
-
Effect of the Microbiome on Intestinal Innate Immune Development in Early Life and the Potential Strategy of Early Intervention.Front Immunol. 2022 Jul 19;13:936300. doi: 10.3389/fimmu.2022.936300. eCollection 2022. Front Immunol. 2022. PMID: 35928828 Free PMC article. Review.
-
GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis.J Immunol. 2018 Mar 1;200(5):1781-1789. doi: 10.4049/jimmunol.1700604. Epub 2018 Jan 31. J Immunol. 2018. PMID: 29386257 Free PMC article.
-
Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages.Gut Microbes. 2017 Jul 4;8(4):400-411. doi: 10.1080/19490976.2017.1299846. Epub 2017 Mar 7. Gut Microbes. 2017. PMID: 28267403 Free PMC article.
References
-
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–685. - PubMed
-
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. - PubMed
-
- du Pre MF, Samsom JN. Adaptive T-cell responses regulating oral tolerance to protein antigen. Allergy. 2011;66(4):478–490. - PubMed
-
- Cording S, Wahl B, Kulkarni D, Chopra H, Pezoldt J, Buettner M, et al. The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 2014;7(2):359–368. - PubMed
-
- Siewert C, Lauer U, Cording S, Bopp T, Schmitt E, Hamann A, et al. Experience-driven development: effector/memory-like alphaE+Foxp3+ regulatory T cells originate from both naive T cells and naturally occurring naive-like regulatory T cells. J Immunol. 2008;180(1):146–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

