Production of Infectious Dengue Virus in Aedes aegypti Is Dependent on the Ubiquitin Proteasome Pathway
- PMID: 26566123
- PMCID: PMC4643912
- DOI: 10.1371/journal.pntd.0004227
Production of Infectious Dengue Virus in Aedes aegypti Is Dependent on the Ubiquitin Proteasome Pathway
Abstract
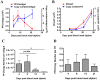
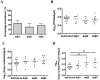
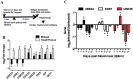
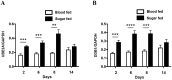
Dengue virus (DENV) relies on host factors to complete its life cycle in its mosquito host for subsequent transmission to humans. DENV first establishes infection in the midgut of Aedes aegypti and spreads to various mosquito organs for lifelong infection. Curiously, studies have shown that infectious DENV titers peak and decrease thereafter in the midgut despite relatively stable viral genome levels. However, the mechanisms that regulate this decoupling of infectious virion production from viral RNA replication have never been determined. We show here that the ubiquitin proteasome pathway (UPP) plays an important role in regulating infectious DENV production. Using RNA interference studies, we show in vivo that knockdown of selected UPP components reduced infectious virus production without altering viral RNA replication in the midgut. Furthermore, this decoupling effect could also be observed after RNAi knockdown in the head/thorax of the mosquito, which otherwise showed direct correlation between infectious DENV titer and viral RNA levels. The dependence on the UPP for successful DENV production is further reinforced by the observed up-regulation of key UPP molecules upon DENV infection that overcome the relatively low expression of these genes after a blood meal. Collectively, our findings indicate an important role for the UPP in regulating DENV production in the mosquito vector.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Proteasome Inhibition Suppresses Dengue Virus Egress in Antibody Dependent Infection.PLoS Negl Trop Dis. 2015 Nov 13;9(11):e0004058. doi: 10.1371/journal.pntd.0004058. eCollection 2015 Nov. PLoS Negl Trop Dis. 2015. PMID: 26565697 Free PMC article.
-
A novel mosquito ubiquitin targets viral envelope protein for degradation and reduces virion production during dengue virus infection.Biochim Biophys Acta. 2016 Sep;1860(9):1898-909. doi: 10.1016/j.bbagen.2016.05.033. Epub 2016 May 27. Biochim Biophys Acta. 2016. PMID: 27241849 Free PMC article.
-
Individual co-variation between viral RNA load and gene expression reveals novel host factors during early dengue virus infection of the Aedes aegypti midgut.PLoS Negl Trop Dis. 2017 Dec 19;11(12):e0006152. doi: 10.1371/journal.pntd.0006152. eCollection 2017 Dec. PLoS Negl Trop Dis. 2017. PMID: 29261661 Free PMC article.
-
The Mosquito Immune System and the Life of Dengue Virus: What We Know and Do Not Know.Pathogens. 2019 Jun 13;8(2):77. doi: 10.3390/pathogens8020077. Pathogens. 2019. PMID: 31200426 Free PMC article. Review.
-
Role of the ubiquitin-proteasome in protein quality control and signaling: implication in the pathogenesis of eye diseases.Prog Mol Biol Transl Sci. 2012;109:347-96. doi: 10.1016/B978-0-12-397863-9.00010-9. Prog Mol Biol Transl Sci. 2012. PMID: 22727427 Review.
Cited by
-
TRIM Proteins in Host Defense and Viral Pathogenesis.Curr Clin Microbiol Rep. 2020;7(4):101-114. doi: 10.1007/s40588-020-00150-8. Epub 2020 Aug 8. Curr Clin Microbiol Rep. 2020. PMID: 32837832 Free PMC article. Review.
-
Influence of Host Blood Meal Source on Gut Microbiota of Wild Caught Aedes aegypti, a Dominant Arboviral Disease Vector.Microorganisms. 2022 Feb 1;10(2):332. doi: 10.3390/microorganisms10020332. Microorganisms. 2022. PMID: 35208787 Free PMC article.
-
Genomic approaches for understanding dengue: insights from the virus, vector, and host.Genome Biol. 2016 Mar 2;17:38. doi: 10.1186/s13059-016-0907-2. Genome Biol. 2016. PMID: 26931545 Free PMC article. Review.
-
Alteration in the Culex pipiens transcriptome reveals diverse mechanisms of the mosquito immune system implicated upon Rift Valley fever phlebovirus exposure.PLoS Negl Trop Dis. 2020 Dec 10;14(12):e0008870. doi: 10.1371/journal.pntd.0008870. eCollection 2020 Dec. PLoS Negl Trop Dis. 2020. PMID: 33301456 Free PMC article.
-
Pathogenesis of Zika Virus Infection.Annu Rev Pathol. 2023 Jan 24;18:181-203. doi: 10.1146/annurev-pathmechdis-031521-034739. Epub 2022 Sep 23. Annu Rev Pathol. 2023. PMID: 36151059 Free PMC article. Review.
References
-
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, et al. (2014) Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384: 1358–1365. 10.1016/S0140-6736(14)61060-6 - DOI - PubMed
-
- Gubler DJ (1989) Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Charles Franklin Craig Lecture. Am J Trop Med Hyg 40: 571–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

