Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update
- PMID: 26563120
- PMCID: PMC4722087
- DOI: 10.1007/s12072-015-9675-4
Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update
Abstract
Worldwide, some 240 million people have chronic hepatitis B virus (HBV), with the highest rates of infection in Africa and Asia. Our understanding of the natural history of HBV infection and the potential for therapy of the resultant disease is continuously improving. New data have become available since the previous APASL guidelines for management of HBV infection were published in 2012. The objective of this manuscript is to update the recommendations for the optimal management of chronic HBV infection. The 2015 guidelines were developed by a panel of Asian experts chosen by the APASL. The clinical practice guidelines are based on evidence from existing publications or, if evidence was unavailable, on the experts' personal experience and opinion after deliberations. Manuscripts and abstracts of important meetings published through January 2015 have been evaluated. This guideline covers the full spectrum of care of patients infected with hepatitis B, including new terminology, natural history, screening, vaccination, counseling, diagnosis, assessment of the stage of liver disease, the indications, timing, choice and duration of single or combination of antiviral drugs, screening for HCC, management in special situations like childhood, pregnancy, coinfections, renal impairment and pre- and post-liver transplant, and policy guidelines. However, areas of uncertainty still exist, and clinicians, patients, and public health authorities must therefore continue to make choices on the basis of the evolving evidence. The final clinical practice guidelines and recommendations are presented here, along with the relevant background information.
Keywords: Acute hepatitis; Guidelines; HBV.
Figures
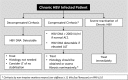
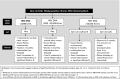




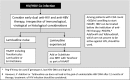
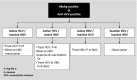
Similar articles
-
Asian Pacific association for the study of liver (APASL) guidelines: hepatitis B virus in pregnancy.Hepatol Int. 2022 Apr;16(2):211-253. doi: 10.1007/s12072-021-10285-5. Epub 2022 Feb 3. Hepatol Int. 2022. PMID: 35113359 Review.
-
Taiwan consensus statement on the management of chronic hepatitis B.J Formos Med Assoc. 2019 Jan;118(1 Pt 1):7-38. doi: 10.1016/j.jfma.2018.11.008. Epub 2018 Dec 6. J Formos Med Assoc. 2019. PMID: 30527436
-
Asian consensus recommendations on optimizing the diagnosis and initiation of treatment of hepatitis B virus infection in resource-limited settings.J Viral Hepat. 2020 May;27(5):466-475. doi: 10.1111/jvh.13244. Epub 2019 Dec 23. J Viral Hepat. 2020. PMID: 31785182
-
NIH consensus development statement on management of hepatitis B.NIH Consens State Sci Statements. 2008 Oct 22-24;25(2):1-29. NIH Consens State Sci Statements. 2008. PMID: 18949020
-
Hepatitis B virus: from diagnosis to treatment.Pathol Biol (Paris). 2010 Aug;58(4):245-53. doi: 10.1016/j.patbio.2010.05.002. Epub 2010 Jul 1. Pathol Biol (Paris). 2010. PMID: 20580167 Review.
Cited by
-
Sensing of Liver-Derived Nicotinamide by Intestinal Group 2 Innate Lymphoid Cells Links Liver Cirrhosis and Ulcerative Colitis Susceptibility.Adv Sci (Weinh). 2024 Oct;11(38):e2404274. doi: 10.1002/advs.202404274. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39119946 Free PMC article.
-
Incidence of Hepatocellular Carcinoma and Decompensated Liver Cirrhosis and Prognostic Accuracy of the PAGE-B HCC Risk Score in a Low Endemic Hepatitis B Virus Infected Population.J Hepatocell Carcinoma. 2022 Oct 18;9:1093-1104. doi: 10.2147/JHC.S372571. eCollection 2022. J Hepatocell Carcinoma. 2022. PMID: 36281336 Free PMC article.
-
Pattern and impact of hepatic adverse events encountered during immune checkpoint inhibitors - A territory-wide cohort study.Cancer Med. 2020 Oct;9(19):7052-7061. doi: 10.1002/cam4.3378. Epub 2020 Aug 11. Cancer Med. 2020. PMID: 32780516 Free PMC article.
-
Current tests for diagnosis of hepatitis B virus infection and immune responses of HBV-related HCC.Front Oncol. 2023 Nov 28;13:1185142. doi: 10.3389/fonc.2023.1185142. eCollection 2023. Front Oncol. 2023. PMID: 38090482 Free PMC article. Review.
-
The effect of a pay-for-performance program on health-related quality of life for patients with hepatitis in Taiwan.Health Qual Life Outcomes. 2022 Sep 5;20(1):130. doi: 10.1186/s12955-022-02038-1. Health Qual Life Outcomes. 2022. PMID: 36064530 Free PMC article.
References
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. - DOI - PMC - PubMed
-
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

