Generation of enterocyte-like cells from human induced pluripotent stem cells for drug absorption and metabolism studies in human small intestine
- PMID: 26559489
- PMCID: PMC4642303
- DOI: 10.1038/srep16479
Generation of enterocyte-like cells from human induced pluripotent stem cells for drug absorption and metabolism studies in human small intestine
Abstract
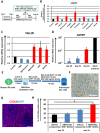
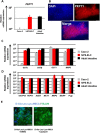
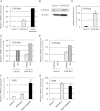
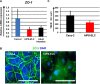
Enterocytes play an important role in drug absorption and metabolism. However, a widely used enterocyte model, Caco-2 cell, has difficulty in evaluating both drug absorption and metabolism because the expression levels of some drug absorption and metabolism-related genes in these cells differ largely from those of human enterocytes. Therefore, we decided to generate the enterocyte-like cells from human induced pluripotent stem (iPS) cells (hiPS-ELCs), which are applicable to drug absorption and metabolism studies. The efficiency of enterocyte differentiation from human iPS cells was significantly improved by using EGF, SB431542, and Wnt3A, and extending the differentiation period. The gene expression levels of cytochrome P450 3A4 (CYP3A4) and peptide transporter 1 in the hiPS-ELCs were higher than those in Caco-2 cells. In addition, CYP3A4 expression in the hiPS-ELCs was induced by treatment with 1, 25-dihydroxyvitamin D3 or rifampicin, which are known to induce CYP3A4 expression, indicating that the hiPS-ELCs have CYP3A4 induction potency. Moreover, the transendothelial electrical resistance (TEER) value of the hiPS-ELC monolayer was approximately 240 Ω*cm(2), suggesting that the hiPS-ELC monolayer could form a barrier. In conclusion, we succeeded in establishing an enterocyte model from human iPS cells which have potential to be applied for drug absorption and metabolism studies.
Figures
Similar articles
-
Functional intestinal monolayers from organoids derived from human iPS cells for drug discovery research.Stem Cell Res Ther. 2024 Feb 29;15(1):57. doi: 10.1186/s13287-024-03685-5. Stem Cell Res Ther. 2024. PMID: 38424603 Free PMC article.
-
Modeling of drug-mediated CYP3A4 induction by using human iPS cell-derived enterocyte-like cells.Biochem Biophys Res Commun. 2016 Apr 15;472(4):631-6. doi: 10.1016/j.bbrc.2016.03.012. Epub 2016 Mar 8. Biochem Biophys Res Commun. 2016. PMID: 26966071
-
Characteristic Analysis of Intestinal Transport in Enterocyte-Like Cells Differentiated from Human Induced Pluripotent Stem Cells.Drug Metab Dispos. 2016 Oct;44(10):0. doi: 10.1124/dmd.116.069336. Epub 2016 Jul 14. Drug Metab Dispos. 2016. PMID: 27417181
-
iPSC-Derived Enterocyte-like Cells for Drug Absorption and Metabolism Studies.Trends Mol Med. 2018 Aug;24(8):696-708. doi: 10.1016/j.molmed.2018.06.001. Epub 2018 Jun 26. Trends Mol Med. 2018. PMID: 29945758 Review.
-
Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling.J Mol Cell Cardiol. 2013 Sep;62:43-50. doi: 10.1016/j.yjmcc.2013.04.022. Epub 2013 Apr 30. J Mol Cell Cardiol. 2013. PMID: 23643470 Review.
Cited by
-
Generation of Caco-2 cells stably expressing CYP3A4·POR·UGT1A1 and CYP3A4·POR·UGT1A1*6 using a PITCh system.Arch Toxicol. 2022 Feb;96(2):499-510. doi: 10.1007/s00204-021-03175-0. Epub 2021 Oct 16. Arch Toxicol. 2022. PMID: 34654938
-
The development of a functional human small intestinal epithelium model for drug absorption.Sci Adv. 2021 Jun 2;7(23):eabh1586. doi: 10.1126/sciadv.abh1586. Print 2021 Jun. Sci Adv. 2021. PMID: 34078609 Free PMC article.
-
Functional intestinal monolayers from organoids derived from human iPS cells for drug discovery research.Stem Cell Res Ther. 2024 Feb 29;15(1):57. doi: 10.1186/s13287-024-03685-5. Stem Cell Res Ther. 2024. PMID: 38424603 Free PMC article.
-
Induced pluripotent stem cells for therapy personalization in pediatric patients: Focus on drug-induced adverse events.World J Stem Cells. 2019 Dec 26;11(12):1020-1044. doi: 10.4252/wjsc.v11.i12.1020. World J Stem Cells. 2019. PMID: 31875867 Free PMC article. Review.
-
Vinblastine treatment decreases the undifferentiated cell contamination of human iPSC-derived intestinal epithelial-like cells.Mol Ther Methods Clin Dev. 2021 Jan 20;20:463-472. doi: 10.1016/j.omtm.2021.01.005. eCollection 2021 Mar 12. Mol Ther Methods Clin Dev. 2021. PMID: 33614822 Free PMC article.
References
-
- Frank R. & Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov 2, 566–580 (2003). - PubMed
-
- Doherty M. M. & Charman W. N. The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism ? Clin Pharmacokinet 41, 235–253 (2002). - PubMed
-
- Leibach F. H. & Ganapathy V. Peptide transporters in the intestine and the kidney. Annu Rev Nutr 16, 99–119 (1996). - PubMed
-
- Kolars J. C., Awni W. M., Merion R. M. & Watkins P. B. First-pass metabolism of cyclosporin by the gut. Lancet 338, 1488–1490 (1991). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

