3D Analysis of HCMV Induced-Nuclear Membrane Structures by FIB/SEM Tomography: Insight into an Unprecedented Membrane Morphology
- PMID: 26556360
- PMCID: PMC4664973
- DOI: 10.3390/v7112900
3D Analysis of HCMV Induced-Nuclear Membrane Structures by FIB/SEM Tomography: Insight into an Unprecedented Membrane Morphology
Abstract
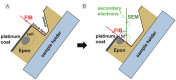
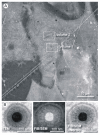
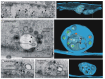
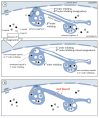
We show that focused ion beam/scanning electron microscopy (FIB/SEM) tomography is an excellent method to analyze the three-dimensional structure of a fibroblast nucleus infected with human cytomegalovirus (HCMV). We found that the previously described infoldings of the inner nuclear membrane, which are unique among its kind, form an extremely complex network of membrane structures not predictable by previous two-dimensional studies. In all cases they contained further invaginations (2nd and 3rd order infoldings). Quantification revealed 5498HCMV capsids within two nuclear segments, allowing an estimate of 15,000 to 30,000 capsids in the entire nucleus five days post infection. Only 0.8% proved to be enveloped capsids which were exclusively detected in 1st order infoldings (perinuclear space). Distribution of the capsids between 1st, 2nd and 3rd order infoldings is in complete agreement with the envelopment/de-envelopment model for egress of HCMV capsids from the nucleus and we confirm that capsid budding does occur at the large infoldings. Based on our results we propose the pushing membrane model: HCMV infection induces local disruption of the nuclear lamina and synthesis of new membrane material which is pushed into the nucleoplasm, forming complex membrane infoldings in a highly abundant manner, which then may be also used by nucleocapsids for budding.
Keywords: FIB/SEM tomography; HCMV; high-pressure freezing; inner nuclear membrane infoldings; nuclear capsid egress; three-dimensional structure.
Figures



Similar articles
-
Cytomegalovirus primary envelopment occurs at large infoldings of the inner nuclear membrane.J Virol. 2007 Mar;81(6):3042-8. doi: 10.1128/JVI.01564-06. Epub 2006 Dec 27. J Virol. 2007. PMID: 17192309 Free PMC article.
-
Cytomegalovirus primary envelopment at large nuclear membrane infoldings: what's new?J Virol. 2007 Jul;81(13):7320-1; author reply 7321-2. doi: 10.1128/JVI.00503-07. J Virol. 2007. PMID: 17567708 Free PMC article. No abstract available.
-
Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97.Viruses. 2018 Jan 13;10(1):35. doi: 10.3390/v10010035. Viruses. 2018. PMID: 29342872 Free PMC article.
-
Venture from the Interior-Herpesvirus pUL31 Escorts Capsids from Nucleoplasmic Replication Compartments to Sites of Primary Envelopment at the Inner Nuclear Membrane.Cells. 2017 Nov 25;6(4):46. doi: 10.3390/cells6040046. Cells. 2017. PMID: 29186822 Free PMC article. Review.
-
The way out: what we know and do not know about herpesvirus nuclear egress.Cell Microbiol. 2013 Feb;15(2):170-8. doi: 10.1111/cmi.12044. Epub 2012 Nov 7. Cell Microbiol. 2013. PMID: 23057731 Review.
Cited by
-
Viral use and subversion of membrane organization and trafficking.J Cell Sci. 2021 Mar 4;134(5):jcs252676. doi: 10.1242/jcs.252676. J Cell Sci. 2021. PMID: 33664154 Free PMC article. Review.
-
The absence of p53 during Human Cytomegalovirus infection leads to decreased UL53 expression, disrupting UL50 localization to the inner nuclear membrane, and thereby inhibiting capsid nuclear egress.Virology. 2016 Oct;497:262-278. doi: 10.1016/j.virol.2016.07.020. Epub 2016 Aug 4. Virology. 2016. PMID: 27498409 Free PMC article.
-
The Role of Lamins in the Nucleoplasmic Reticulum, a Pleiomorphic Organelle That Enhances Nucleo-Cytoplasmic Interplay.Front Cell Dev Biol. 2022 Jun 16;10:914286. doi: 10.3389/fcell.2022.914286. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35784476 Free PMC article. Review.
-
The human cytomegalovirus decathlon: Ten critical replication events provide opportunities for restriction.Front Cell Dev Biol. 2022 Nov 25;10:1053139. doi: 10.3389/fcell.2022.1053139. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36506089 Free PMC article. Review.
-
Properties of Oligomeric Interaction of the Cytomegalovirus Core Nuclear Egress Complex (NEC) and Its Sensitivity to an NEC Inhibitory Small Molecule.Viruses. 2021 Mar 11;13(3):462. doi: 10.3390/v13030462. Viruses. 2021. PMID: 33799898 Free PMC article.
References
-
- Homman-Loudiyi M., Hultenby K., Britt W., Soderberg-Naucler C. Envelopment of Human Cytomegalovirus Occurs by Budding into Golgi-Derived Vacuole Compartments Positive for gB, Rab 3, Trans-Golgi Network 46, and Mannosidase II. J. Virol. 2003;77:3191–3203. doi: 10.1128/JVI.77.5.3191-3203.2003. - DOI - PMC - PubMed
-
- Jiang X.J., Adler B., Sampaio K.L., Digel M., Jahn G., Ettischer N., Stierhof Y.-D., Scrivano L., Koszinowski U., Mach M., et al. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J. Virol. 2008;82:2802–2812. doi: 10.1128/JVI.01550-07. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

