Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials
- PMID: 26556276
- PMCID: PMC4659470
- DOI: 10.1128/mBio.01697-15
Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials
Abstract
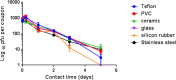
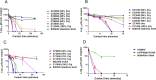
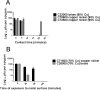
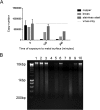
The evolution of new and reemerging historic virulent strains of respiratory viruses from animal reservoirs is a significant threat to human health. Inefficient human-to-human transmission of zoonotic strains may initially limit the spread of transmission, but an infection may be contracted by touching contaminated surfaces. Enveloped viruses are often susceptible to environmental stresses, but the human coronaviruses responsible for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) have recently caused increasing concern of contact transmission during outbreaks. We report here that pathogenic human coronavirus 229E remained infectious in a human lung cell culture model following at least 5 days of persistence on a range of common nonbiocidal surface materials, including polytetrafluoroethylene (Teflon; PTFE), polyvinyl chloride (PVC), ceramic tiles, glass, silicone rubber, and stainless steel. We have shown previously that noroviruses are destroyed on copper alloy surfaces. In this new study, human coronavirus 229E was rapidly inactivated on a range of copper alloys (within a few minutes for simulated fingertip contamination) and Cu/Zn brasses were very effective at lower copper concentration. Exposure to copper destroyed the viral genomes and irreversibly affected virus morphology, including disintegration of envelope and dispersal of surface spikes. Cu(I) and Cu(II) moieties were responsible for the inactivation, which was enhanced by reactive oxygen species generation on alloy surfaces, resulting in even faster inactivation than was seen with nonenveloped viruses on copper. Consequently, copper alloy surfaces could be employed in communal areas and at any mass gatherings to help reduce transmission of respiratory viruses from contaminated surfaces and protect the public health.
Importance: Respiratory viruses are responsible for more deaths globally than any other infectious agent. Animal coronaviruses that "host jump" to humans result in severe infections with high mortality, such as severe acute respiratory syndrome (SARS) and, more recently, Middle East respiratory syndrome (MERS). We show here that a closely related human coronavirus, 229E, which causes upper respiratory tract infection in healthy individuals and serious disease in patients with comorbidities, remained infectious on surface materials common to public and domestic areas for several days. The low infectious dose means that this is a significant infection risk to anyone touching a contaminated surface. However, rapid inactivation, irreversible destruction of viral RNA, and massive structural damage were observed in coronavirus exposed to copper and copper alloy surfaces. Incorporation of copper alloy surfaces in conjunction with effective cleaning regimens and good clinical practice could help to control transmission of respiratory coronaviruses, including MERS and SARS.
Copyright © 2015 Warnes et al.
Figures


Similar articles
-
Inactivation of murine norovirus on a range of copper alloy surfaces is accompanied by loss of capsid integrity.Appl Environ Microbiol. 2015 Feb;81(3):1085-91. doi: 10.1128/AEM.03280-14. Epub 2014 Dec 1. Appl Environ Microbiol. 2015. PMID: 25452290 Free PMC article.
-
Assessment of Antiviral Coatings for High-Touch Surfaces by Using Human Coronaviruses HCoV-229E and SARS-CoV-2.Appl Environ Microbiol. 2021 Sep 10;87(19):e0109821. doi: 10.1128/AEM.01098-21. Epub 2021 Sep 10. Appl Environ Microbiol. 2021. PMID: 34288707 Free PMC article.
-
Inactivation of norovirus on dry copper alloy surfaces.PLoS One. 2013 Sep 9;8(9):e75017. doi: 10.1371/journal.pone.0075017. eCollection 2013. PLoS One. 2013. PMID: 24040380 Free PMC article.
-
Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination.J Hosp Infect. 2016 Mar;92(3):235-50. doi: 10.1016/j.jhin.2015.08.027. Epub 2015 Oct 3. J Hosp Infect. 2016. PMID: 26597631 Free PMC article. Review.
-
Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents.J Hosp Infect. 2020 Mar;104(3):246-251. doi: 10.1016/j.jhin.2020.01.022. Epub 2020 Feb 6. J Hosp Infect. 2020. PMID: 32035997 Free PMC article. Review.
Cited by
-
Evaluation of the persistence of SARS-CoV-2 (ATCC® VR-1986HK™) on two different food contact materials: flow pack polyethylene and polystyrene food trays.Lebensm Wiss Technol. 2021 Jul;146:111606. doi: 10.1016/j.lwt.2021.111606. Epub 2021 May 4. Lebensm Wiss Technol. 2021. PMID: 33967345 Free PMC article.
-
Fight against COVID-19: The case of antiviral surfaces.APL Mater. 2021 Mar 1;9(3):031112. doi: 10.1063/5.0043009. APL Mater. 2021. PMID: 33842101 Free PMC article.
-
Biomedical Science to Tackle the COVID-19 Pandemic: Current Status and Future Perspectives.Molecules. 2020 Oct 11;25(20):4620. doi: 10.3390/molecules25204620. Molecules. 2020. PMID: 33050601 Free PMC article. Review.
-
In vitro assessment of antibacterial and antiviral activity of three copper products after 200 rounds of simulated use.Biometals. 2024 Aug;37(4):849-856. doi: 10.1007/s10534-023-00572-z. Epub 2023 Dec 22. Biometals. 2024. PMID: 38133868 Free PMC article.
-
Passive antifouling and active self-disinfecting antiviral surfaces.Chem Eng J. 2022 Oct 15;446:137048. doi: 10.1016/j.cej.2022.137048. Epub 2022 May 18. Chem Eng J. 2022. PMID: 35601363 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
