Engineered Mammalian RNAi Can Elicit Antiviral Protection that Negates the Requirement for the Interferon Response
- PMID: 26549455
- PMCID: PMC4654977
- DOI: 10.1016/j.celrep.2015.10.020
Engineered Mammalian RNAi Can Elicit Antiviral Protection that Negates the Requirement for the Interferon Response
Abstract
Although the intrinsic antiviral cell defenses of many kingdoms utilize pathogen-specific small RNAs, the antiviral response of chordates is primarily protein based and not uniquely tailored to the incoming microbe. In an effort to explain this evolutionary bifurcation, we determined whether antiviral RNAi was sufficient to replace the protein-based type I interferon (IFN-I) system of mammals. To this end, we recreated an RNAi-like response in mammals and determined its effectiveness to combat influenza A virus in vivo in the presence and absence of the canonical IFN-I system. Mammalian antiviral RNAi, elicited by either host- or virus-derived small RNAs, effectively attenuated virus and prevented disease independently of the innate immune response. These data find that chordates could have utilized RNAi as their primary antiviral cell defense and suggest that the IFN-I system emerged as a result of natural selection imposed by ancient pathogens.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
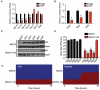
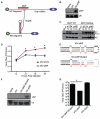
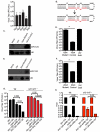
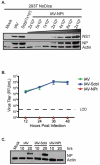
Similar articles
-
Engineered virus-encoded pre-microRNA (pre-miRNA) induces sequence-specific antiviral response in addition to nonspecific immunity in a fish cell line: convergence of RNAi-related pathways and IFN-related pathways in antiviral response.Antiviral Res. 2008 Dec;80(3):316-23. doi: 10.1016/j.antiviral.2008.07.005. Epub 2008 Aug 5. Antiviral Res. 2008. PMID: 18687362
-
Antiviral innate immune response of RNA interference.J Infect Dev Ctries. 2014 Jul 14;8(7):804-10. doi: 10.3855/jidc.4187. J Infect Dev Ctries. 2014. PMID: 25022288 Review.
-
ADAR1 Biology Can Hinder Effective Antiviral RNA Interference.J Virol. 2023 Apr 27;97(4):e0024523. doi: 10.1128/jvi.00245-23. Epub 2023 Apr 5. J Virol. 2023. PMID: 37017521 Free PMC article.
-
Interferons and microRNAs.J Interferon Cytokine Res. 2010 Nov;30(11):825-8. doi: 10.1089/jir.2010.0080. Epub 2010 Oct 12. J Interferon Cytokine Res. 2010. PMID: 20939680 Review.
-
The Nucleocapsid Protein of Coronaviruses Acts as a Viral Suppressor of RNA Silencing in Mammalian Cells.J Virol. 2015 Sep;89(17):9029-43. doi: 10.1128/JVI.01331-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085159 Free PMC article.
Cited by
-
Slicing and dicing viruses: antiviral RNA interference in mammals.EMBO J. 2019 Apr 15;38(8):e100941. doi: 10.15252/embj.2018100941. Epub 2019 Mar 14. EMBO J. 2019. PMID: 30872283 Free PMC article. Review.
-
Antiviral Defense and Innate Immune Memory in the Oyster.Viruses. 2018 Mar 16;10(3):133. doi: 10.3390/v10030133. Viruses. 2018. PMID: 29547519 Free PMC article. Review.
-
The evolving world of small RNAs from RNA viruses.Wiley Interdiscip Rev RNA. 2016 Sep;7(5):575-88. doi: 10.1002/wrna.1351. Epub 2016 Apr 5. Wiley Interdiscip Rev RNA. 2016. PMID: 27046163 Free PMC article. Review.
-
Antiviral RNAi in Insects and Mammals: Parallels and Differences.Viruses. 2019 May 16;11(5):448. doi: 10.3390/v11050448. Viruses. 2019. PMID: 31100912 Free PMC article. Review.
-
Ancient viral integrations in marsupials: a potential antiviral defence.Virus Evol. 2021 Sep 2;7(2):veab076. doi: 10.1093/ve/veab076. eCollection 2021. Virus Evol. 2021. PMID: 34548931 Free PMC article.
References
-
- Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–764. - PubMed
-
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

