The Stereociliary Paracrystal Is a Dynamic Cytoskeletal Scaffold In Vivo
- PMID: 26549442
- PMCID: PMC4654971
- DOI: 10.1016/j.celrep.2015.10.003
The Stereociliary Paracrystal Is a Dynamic Cytoskeletal Scaffold In Vivo
Abstract
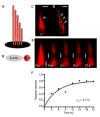
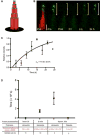
Permanency of mechanosensory stereocilia may be the consequence of low protein turnover or rapid protein renewal. Here, we devise a system, using optical techniques in live zebrafish, to distinguish between these mechanisms. We demonstrate that the stereocilium's abundant actin cross-linker fascin 2b exchanges, without bias or a phosphointermediate, orders of magnitude faster (t1/2 of 76.3 s) than any other known hair bundle protein. To establish the logic of fascin 2b's exchange, we examine whether filamentous actin is dynamic and detect substantial β-actin exchange within the stereocilium's paracrystal (t1/2 of 4.08 hr). We propose that fascin 2b's behavior may enable cross-linking at fast timescales of stereocilia vibration while noninstructively facilitating the slower process of actin exchange. Furthermore, tip protein myosin XVa fully exchanges in hours (t1/2 of 11.6 hr), indicating that delivery of myosin-associated cargo occurs in mature stereocilia. These findings suggest that stereocilia permanency is underpinned by vibrant protein exchange.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal.J Cell Biol. 2004 Mar 15;164(6):887-97. doi: 10.1083/jcb.200310055. J Cell Biol. 2004. PMID: 15024034 Free PMC article.
-
Fascin 2b is a component of stereocilia that lengthens actin-based protrusions.PLoS One. 2011;6(4):e14807. doi: 10.1371/journal.pone.0014807. Epub 2011 Apr 26. PLoS One. 2011. PMID: 21625653 Free PMC article.
-
The stable actin core of mechanosensory stereocilia features continuous turnover of actin cross-linkers.Mol Biol Cell. 2018 Aug 1;29(15):1856-1865. doi: 10.1091/mbc.E18-03-0196. Epub 2018 Jun 6. Mol Biol Cell. 2018. PMID: 29874122 Free PMC article.
-
When size matters: the dynamic regulation of stereocilia lengths.Curr Opin Cell Biol. 2005 Feb;17(1):55-61. doi: 10.1016/j.ceb.2004.12.005. Curr Opin Cell Biol. 2005. PMID: 15661519 Review.
-
Stereocilia morphogenesis and maintenance through regulation of actin stability.Semin Cell Dev Biol. 2017 May;65:88-95. doi: 10.1016/j.semcdb.2016.08.017. Epub 2016 Aug 23. Semin Cell Dev Biol. 2017. PMID: 27565685 Free PMC article. Review.
Cited by
-
A cryo-tomography-based volumetric model of the actin core of mouse vestibular hair cell stereocilia lacking plastin 1.J Struct Biol. 2020 Apr 1;210(1):107461. doi: 10.1016/j.jsb.2020.107461. Epub 2020 Jan 18. J Struct Biol. 2020. PMID: 31962158 Free PMC article.
-
The actin cytoskeleton in hair bundle development and hearing loss.Hear Res. 2023 Sep 1;436:108817. doi: 10.1016/j.heares.2023.108817. Epub 2023 May 26. Hear Res. 2023. PMID: 37300948 Free PMC article. Review.
-
IKKε inhibits PKC to promote Fascin-dependent actin bundling.Development. 2016 Oct 15;143(20):3806-3816. doi: 10.1242/dev.138495. Epub 2016 Aug 30. Development. 2016. PMID: 27578797 Free PMC article.
-
Supervillin Is a Component of the Hair Cell's Cuticular Plate and the Head Plates of Organ of Corti Supporting Cells.PLoS One. 2016 Jul 14;11(7):e0158349. doi: 10.1371/journal.pone.0158349. eCollection 2016. PLoS One. 2016. PMID: 27415442 Free PMC article.
-
Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like.Nat Commun. 2016 Mar 1;7:10833. doi: 10.1038/ncomms10833. Nat Commun. 2016. PMID: 26926603 Free PMC article.
References
-
- Anderson DW, Probst FJ, Belyantseva IA, Fridell RA, Beyer L, Martin DM, Wu D, Kachar B, Friedman TB, Raphael Y, et al. The motor and tail regions of myosin XV are critical for normal structure and function of auditory and vestibular hair cells. Hum Mol Genet. 2000;9:1729–1738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

