Increased pretreatment serum IFN-β/α ratio predicts non-response to tumour necrosis factor α inhibition in rheumatoid arthritis
- PMID: 26546586
- PMCID: PMC4860184
- DOI: 10.1136/annrheumdis-2015-208001
Increased pretreatment serum IFN-β/α ratio predicts non-response to tumour necrosis factor α inhibition in rheumatoid arthritis
Abstract
Objective: Studies suggest that circulating type I interferon (IFN) may predict response to biological agents in rheumatoid arthritis (RA). Prediction of response prior to initiating therapy would represent a major advancement.
Methods: We studied sera from a test set of 32 patients with RA from the Auto-immune Biomarkers Collaborative Network Consortium and a validation set of 92 patients with RA from the Treatment Efficacy and Toxicity in Rheumatoid Arthritis Database and Repository registry. The test set included those with good response or no response to tumour necrosis factor (TNF) inhibitors at 14 weeks by European League Against Rheumatism criteria. The validation set included subjects with good, moderate or no response at 12 weeks. Total serum type I IFN activity, IFN-α and IFN-β activity were measured using a functional reporter cell assay.
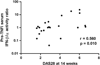
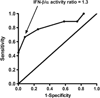
Results: In the test set, an increased ratio of IFN-β to IFN-α (IFN-β/α activity ratio) in pretreatment serum associated with lack of response to TNF inhibition (p=0.013). Anti-cyclic citrullinated peptide antibody titre and class of TNF inhibitor did not influence this relationship. A receiver-operator curve supported a ratio of 1.3 as the optimal cut-off. In the validation set, subjects with an IFN-β/α activity ratio >1.3 were significantly more likely to have non-response than good response (OR=6.67, p=0.018). The test had 77% specificity and 45% sensitivity for prediction of non-response compared with moderate or good response. Meta-analysis of test and validation sets confirmed strong predictive capacity of IFN-β/α activity ratio (p=0.005).
Conclusions: Increased pretreatment serum IFN-β/α ratio strongly associated with non-response to TNF inhibition. This study supports further investigation of serum type I IFN in predicting outcome of TNF inhibition in RA.
Keywords: Cytokines; Rheumatoid Arthritis; TNF-alpha; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

Similar articles
-
Association of the response to tumor necrosis factor antagonists with plasma type I interferon activity and interferon-beta/alpha ratios in rheumatoid arthritis patients: a post hoc analysis of a predominantly Hispanic cohort.Arthritis Rheum. 2010 Feb;62(2):392-401. doi: 10.1002/art.27226. Arthritis Rheum. 2010. PMID: 20112385 Free PMC article. Clinical Trial.
-
Interferon gene expression signature in rheumatoid arthritis neutrophils correlates with a good response to TNFi therapy.Rheumatology (Oxford). 2015 Jan;54(1):188-93. doi: 10.1093/rheumatology/keu299. Epub 2014 Aug 13. Rheumatology (Oxford). 2015. PMID: 25125592
-
Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register.Rheumatology (Oxford). 2006 Dec;45(12):1558-65. doi: 10.1093/rheumatology/kel149. Epub 2006 May 16. Rheumatology (Oxford). 2006. PMID: 16705046
-
Rheumatoid factor and response to TNF antagonists in rheumatoid arthritis: systematic review and meta-analysis of observational studies.Joint Bone Spine. 2014 Jan;81(1):41-50. doi: 10.1016/j.jbspin.2013.04.004. Epub 2013 Jun 2. Joint Bone Spine. 2014. PMID: 23731644 Review.
-
Tumour necrosis factor alpha -308G->A polymorphism is not associated with response to TNFalpha blockers in Caucasian patients with rheumatoid arthritis: systematic review and meta-analysis.Ann Rheum Dis. 2010 Jun;69(6):1022-8. doi: 10.1136/ard.2009.117622. Epub 2009 Dec 4. Ann Rheum Dis. 2010. PMID: 19966089 Review.
Cited by
-
Nature of the Association between Rheumatoid Arthritis and Cervical Cancer and Its Potential Therapeutic Implications.Nutrients. 2024 Aug 5;16(15):2569. doi: 10.3390/nu16152569. Nutrients. 2024. PMID: 39125448 Free PMC article. Review.
-
A20: a master regulator of arthritis.Arthritis Res Ther. 2020 Sep 21;22(1):220. doi: 10.1186/s13075-020-02281-1. Arthritis Res Ther. 2020. PMID: 32958016 Free PMC article. Review.
-
Mitochondria in innate immunity signaling and its therapeutic implications in autoimmune diseases.Front Immunol. 2023 Apr 12;14:1160035. doi: 10.3389/fimmu.2023.1160035. eCollection 2023. Front Immunol. 2023. PMID: 37122709 Free PMC article. Review.
-
Distinct Single Cell Gene Expression in Peripheral Blood Monocytes Correlates With Tumor Necrosis Factor Inhibitor Treatment Response Groups Defined by Type I Interferon in Rheumatoid Arthritis.Front Immunol. 2020 Jul 16;11:1384. doi: 10.3389/fimmu.2020.01384. eCollection 2020. Front Immunol. 2020. PMID: 32765497 Free PMC article.
-
Serum level of IFNβ distinguishes early from late relapses after biologics withdrawal in rheumatoid arthritis.Sci Rep. 2022 Oct 3;12(1):16547. doi: 10.1038/s41598-022-21160-0. Sci Rep. 2022. PMID: 36192530 Free PMC article.
References
-
- Finckh A, Liang MH, van Herckenrode CM, et al. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum. 2006;55:864–872. - PubMed
-
- Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48:1771–1774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
