The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds
- PMID: 26543676
- PMCID: PMC4620518
- DOI: 10.1089/wound.2014.0579
The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds
Abstract
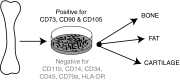
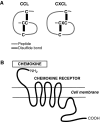
Significance: Mesenchymal stem cells (MSCs) are being administered to cutaneous wounds with the goal of accelerating wound closure and promoting regeneration instead of scar formation. An ongoing challenge for cell-based therapies is achieving effective and optimal targeted delivery and engraftment at the site of injury. Contributing to this challenge is our incomplete understanding of endogenous MSC homing to sites of injury. Recent Advances: Chemokines and their receptors are now recognized as important mediators of stem cell homing. To date, the most studied chemokine-chemokine receptor axis in MSC homing to wounds is CXCL12-CXCR4 but recent work suggests that CCL27-CCR10 and CCL21-CCR7 may also be involved. Critical Issues: Strategies to enhance chemokine-mediated MSC homing to wounds are using a variety of approaches to amplify the chemokine signal at the wound site and/or overexpress specific chemokine receptors on the surface of the MSC. Future Directions: Harnessing chemokine signaling may enhance the therapeutic effects of stem cell therapy by increasing the number of both exogenous and endogenous stem cells recruited to the site of injury. Alternatively, chemokine-based therapies directly targeting endogenous stem cells may circumvent the need for the time-consuming and costly isolation and expansion of autologous stem cells prior to therapeutic administration.
Figures



Similar articles
-
[Chemokine directed homing of transplanted adult stem cells in wound healing and tissue regeneration].Verh Dtsch Ges Pathol. 2004;88:170-3. Verh Dtsch Ges Pathol. 2004. PMID: 16892549 German.
-
Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues.Cytotherapy. 2013 Feb;15(2):171-184.e1. doi: 10.1016/j.jcyt.2012.11.006. Cytotherapy. 2013. PMID: 23321329 Free PMC article.
-
CTACK/CCL27 accelerates skin regeneration via accumulation of bone marrow-derived keratinocytes.Stem Cells. 2006 Dec;24(12):2810-6. doi: 10.1634/stemcells.2006-0264. Epub 2006 Aug 24. Stem Cells. 2006. PMID: 16931770
-
Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy.Cell Biol Int. 2015 Jan;39(1):23-34. doi: 10.1002/cbin.10378. Epub 2014 Oct 13. Cell Biol Int. 2015. PMID: 25231104 Review.
-
Toward in situ tissue engineering: chemokine-guided stem cell recruitment.Trends Biotechnol. 2014 Sep;32(9):483-92. doi: 10.1016/j.tibtech.2014.06.008. Epub 2014 Jul 21. Trends Biotechnol. 2014. PMID: 25059433 Review.
Cited by
-
Biologics and their delivery systems: Trends in myocardial infarction.Adv Drug Deliv Rev. 2021 Jun;173:181-215. doi: 10.1016/j.addr.2021.03.014. Epub 2021 Mar 26. Adv Drug Deliv Rev. 2021. PMID: 33775706 Free PMC article. Review.
-
Preliminary Studies of the Impact of CXCL12 on the Foreign Body Reaction to Pancreatic Islets Microencapsulated in Alginate in Nonhuman Primates.Transplant Direct. 2019 Apr 15;5(5):e447. doi: 10.1097/TXD.0000000000000890. eCollection 2019 May. Transplant Direct. 2019. PMID: 31165082 Free PMC article.
-
Controlled and Sequential Delivery of Stromal Derived Factor-1 α (SDF-1α) and Magnesium Ions from Bifunctional Hydrogel for Bone Regeneration.Polymers (Basel). 2022 Jul 15;14(14):2872. doi: 10.3390/polym14142872. Polymers (Basel). 2022. PMID: 35890649 Free PMC article.
-
Paracrine interleukin-8 affects mesenchymal stem cells through the Akt pathway and enhances human umbilical vein endothelial cell proliferation and migration.Biosci Rep. 2021 May 28;41(5):BSR20210198. doi: 10.1042/BSR20210198. Biosci Rep. 2021. Retraction in: Biosci Rep. 2021 Dec 22;41(12):BSR-2021-0198_RET. doi: 10.1042/BSR-2021-0198_RET PMID: 33843989 Free PMC article. Retracted.
-
Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites.Int J Nanomedicine. 2019 Jan 14;14:573-589. doi: 10.2147/IJN.S184920. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30666115 Free PMC article.
References
-
- Karp JM. and Leng Teo GS: Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, et al. : Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

