A complex of Rab13 with MICAL-L2 and α-actinin-4 is essential for insulin-dependent GLUT4 exocytosis
- PMID: 26538022
- PMCID: PMC4694764
- DOI: 10.1091/mbc.E15-05-0319
A complex of Rab13 with MICAL-L2 and α-actinin-4 is essential for insulin-dependent GLUT4 exocytosis
Abstract
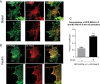
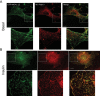
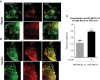
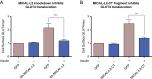
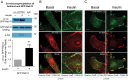
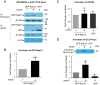
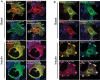
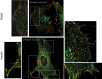
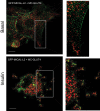
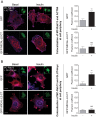
Insulin promotes glucose uptake into skeletal muscle through recruitment of glucose transporter 4 (GLUT4) to the plasma membrane. Rab GTPases are molecular switches mobilizing intracellular vesicles, and Rab13 is necessary for insulin-regulated GLUT4-vesicle exocytic translocation in muscle cells. We show that Rab13 engages the scaffold protein MICAL-L2 in this process. RNA interference-mediated knockdown of MICAL-L2 or truncated MICAL-L2 (MICAL-L2-CT) impaired insulin-stimulated GLUT4 translocation. Insulin increased Rab13 binding to MICAL-L2, assessed by pull down and colocalization under confocal fluorescence and structured illumination microscopies. Association was also visualized at the cell periphery using TIRF microscopy. Insulin further increased binding of MICAL-L2 to α-actinin-4 (ACTN4), a protein involved in GLUT4 translocation. Rab13, MICAL-L2, and ACTN4 formed an insulin-dependent complex assessed by pull down and confocal fluorescence imaging. Of note, GLUT4 associated with the complex in response to insulin, requiring the ACTN4-binding domain in MICAL-L2. This was demonstrated by pull down with distinct fragments of MICAL-L2 and confocal and structured illumination microscopies. Finally, expression of MICAL-L2-CT abrogated the insulin-dependent colocalization of Rab13 with ACTN4 or Rab13 with GLUT4. Our findings suggest that MICAL-L2 is an effector of insulin-activated Rab13, which links to GLUT4 through ACTN4, localizing GLUT4 vesicles at the muscle cell periphery to enable their fusion with the membrane.
© 2016 Sun et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
Similar articles
-
Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells.Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19909-14. doi: 10.1073/pnas.1009523107. Epub 2010 Nov 1. Proc Natl Acad Sci U S A. 2010. PMID: 21041651 Free PMC article.
-
Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions.Mol Cell Biol. 2008 May;28(10):3324-35. doi: 10.1128/MCB.00144-08. Epub 2008 Mar 10. Mol Cell Biol. 2008. PMID: 18332111 Free PMC article.
-
The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions.Mol Biol Cell. 2008 Mar;19(3):971-83. doi: 10.1091/mbc.e07-06-0551. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094055 Free PMC article.
-
Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation.Am J Physiol Cell Physiol. 2014 May 15;306(10):C879-86. doi: 10.1152/ajpcell.00069.2014. Epub 2014 Mar 5. Am J Physiol Cell Physiol. 2014. PMID: 24598362 Review.
-
Update on GLUT4 Vesicle Traffic: A Cornerstone of Insulin Action.Trends Endocrinol Metab. 2017 Aug;28(8):597-611. doi: 10.1016/j.tem.2017.05.002. Epub 2017 Jun 8. Trends Endocrinol Metab. 2017. PMID: 28602209 Review.
Cited by
-
β-catenin regulates muscle glucose transport via actin remodelling and M-cadherin binding.Mol Metab. 2020 Dec;42:101091. doi: 10.1016/j.molmet.2020.101091. Epub 2020 Oct 1. Mol Metab. 2020. PMID: 33011305 Free PMC article.
-
GLUT4 On the move.Biochem J. 2022 Feb 11;479(3):445-462. doi: 10.1042/BCJ20210073. Biochem J. 2022. PMID: 35147164 Free PMC article.
-
AMPK-mediated phosphorylation enhances the auto-inhibition of TBC1D17 to promote Rab5-dependent glucose uptake.Cell Death Differ. 2021 Dec;28(12):3214-3234. doi: 10.1038/s41418-021-00809-9. Epub 2021 May 27. Cell Death Differ. 2021. PMID: 34045668 Free PMC article.
-
Ubiquitin-like processing of TUG proteins as a mechanism to regulate glucose uptake and energy metabolism in fat and muscle.Front Endocrinol (Lausanne). 2022 Sep 29;13:1019405. doi: 10.3389/fendo.2022.1019405. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36246906 Free PMC article. Review.
-
High MICAL-L2 expression and its role in the prognosis of colon adenocarcinoma.BMC Cancer. 2022 May 2;22(1):487. doi: 10.1186/s12885-022-09614-0. BMC Cancer. 2022. PMID: 35501725 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

