Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial
- PMID: 26511996
- PMCID: PMC4893095
- DOI: 10.1136/annrheumdis-2015-207628
Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial
Abstract
Objectives: The efficacy of tocilizumab (TCZ), an anti-interleukin-6 receptor antibody, has not previously been evaluated in a population consisting exclusively of patients with early rheumatoid arthritis (RA).
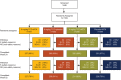
Methods: In a double-blind randomised controlled trial (FUNCTION), 1162 methotrexate (MTX)-naive patients with early progressive RA were randomly assigned (1:1:1:1) to one of four treatment groups: 4 mg/kg TCZ+MTX, 8 mg/kg TCZ+MTX, 8 mg/kg TCZ+placebo and placebo+MTX (comparator group). The primary outcome was remission according to Disease Activity Score using 28 joints (DAS28-erythrocyte sedimentation rate (ESR) <2.6) at week 24. Radiographic and physical function outcomes were also evaluated. We report results through week 52.
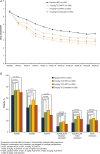
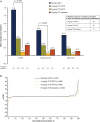
Results: The intent-to-treat population included 1157 patients. Significantly more patients receiving 8 mg/kg TCZ+MTX and 8 mg/kg TCZ+placebo than receiving placebo+MTX achieved DAS28-ESR remission at week 24 (45% and 39% vs 15%; p<0.0001). The 8 mg/kg TCZ+MTX group also achieved significantly greater improvement in radiographic disease progression and physical function at week 52 than did patients treated with placebo+MTX (mean change from baseline in van der Heijde-modified total Sharp score, 0.08 vs 1.14 (p=0.0001); mean reduction in Health Assessment Disability Index, -0.81 vs -0.64 (p=0.0024)). In addition, the 8 mg/kg TCZ+placebo and 4 mg/kg TCZ+MTX groups demonstrated clinical efficacy that was at least as effective as MTX for these key secondary endpoints. Serious adverse events were similar among treatment groups. Adverse events resulting in premature withdrawal occurred in 20% of patients in the 8 mg/kg TCZ+MTX group.
Conclusions: TCZ is effective in combination with MTX and as monotherapy for the treatment of patients with early RA.
Trial registration number: ClinicalTrials.gov, number NCT01007435.
Keywords: DMARDs (biologic); Early Rheumatoid Arthritis; Methotrexate.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

Similar articles
-
Tocilizumab combination therapy or monotherapy or methotrexate monotherapy in methotrexate-naive patients with early rheumatoid arthritis: 2-year clinical and radiographic results from the randomised, placebo-controlled FUNCTION trial.Ann Rheum Dis. 2017 Jul;76(7):1279-1284. doi: 10.1136/annrheumdis-2016-210561. Epub 2017 Apr 7. Ann Rheum Dis. 2017. PMID: 28389552 Free PMC article. Clinical Trial.
-
Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY).Ann Rheum Dis. 2013 Jan;72(1):43-50. doi: 10.1136/annrheumdis-2011-201282. Epub 2012 May 5. Ann Rheum Dis. 2013. PMID: 22562983 Free PMC article. Clinical Trial.
-
Sustained Response Following Discontinuation of Methotrexate in Patients With Rheumatoid Arthritis Treated With Subcutaneous Tocilizumab: Results From a Randomized, Controlled Trial.Arthritis Rheumatol. 2018 Aug;70(8):1200-1208. doi: 10.1002/art.40493. Epub 2018 Jun 14. Arthritis Rheumatol. 2018. PMID: 29575803 Clinical Trial.
-
Systematic review of tocilizumab for rheumatoid arthritis: a new biologic agent targeting the interleukin-6 receptor.Clin Ther. 2012 Apr;34(4):788-802.e3. doi: 10.1016/j.clinthera.2012.02.014. Epub 2012 Mar 22. Clin Ther. 2012. PMID: 22444783 Free PMC article. Review.
-
Meta-Regression of a Dose-Response Relationship of Methotrexate in Mono- and Combination Therapy in Disease-Modifying Antirheumatic Drug-Naive Early Rheumatoid Arthritis Patients.Arthritis Care Res (Hoboken). 2017 Oct;69(10):1473-1483. doi: 10.1002/acr.23164. Epub 2017 Aug 31. Arthritis Care Res (Hoboken). 2017. PMID: 27992656 Review.
Cited by
-
Structural Understanding of Interleukin 6 Family Cytokine Signaling and Targeted Therapies: Focus on Interleukin 11.Front Immunol. 2020 Jul 16;11:1424. doi: 10.3389/fimmu.2020.01424. eCollection 2020. Front Immunol. 2020. PMID: 32765502 Free PMC article. Review.
-
Predicting treatment response to IL6R blockers in rheumatoid arthritis.Rheumatology (Oxford). 2020 Dec 1;59(12):3603-3610. doi: 10.1093/rheumatology/keaa529. Rheumatology (Oxford). 2020. PMID: 32864695 Free PMC article. Review.
-
Follicular helper T cells: potential therapeutic targets in rheumatoid arthritis.Cell Mol Life Sci. 2021 Jun;78(12):5095-5106. doi: 10.1007/s00018-021-03839-1. Epub 2021 Apr 20. Cell Mol Life Sci. 2021. PMID: 33880615 Free PMC article. Review.
-
A systematic literature review informing the consensus statement on efficacy and safety of pharmacological treatment with interleukin-6 pathway inhibition with biological DMARDs in immune-mediated inflammatory diseases.RMD Open. 2022 Sep;8(2):e002359. doi: 10.1136/rmdopen-2022-002359. RMD Open. 2022. PMID: 36260501 Free PMC article.
-
Selectivity of Janus Kinase Inhibitors in Rheumatoid Arthritis and Other Immune-Mediated Inflammatory Diseases: Is Expectation the Root of All Headache?Drugs. 2020 Aug;80(12):1183-1201. doi: 10.1007/s40265-020-01349-1. Drugs. 2020. PMID: 32681420 Free PMC article. Review.
References
-
- Genovese MC, Bathon JM, Fleischmann RM, et al. . Long-term safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol 2005;32:1232–42. - PubMed
-
- Singh JA, Furst DE, Bharat A, et al. . 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res 2012;64:625–39. 10.1002/acr.21641 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
