Mapping the Binding Site of the Inhibitor Tariquidar That Stabilizes the First Transmembrane Domain of P-glycoprotein
- PMID: 26507655
- PMCID: PMC4705942
- DOI: 10.1074/jbc.M115.695171
Mapping the Binding Site of the Inhibitor Tariquidar That Stabilizes the First Transmembrane Domain of P-glycoprotein
Abstract
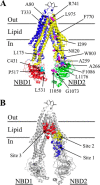
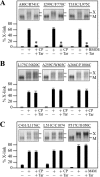
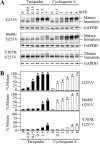
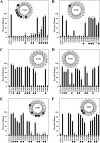
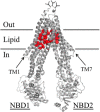
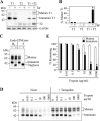
ABC (ATP-binding cassette) transporters are clinically important because drug pumps like P-glycoprotein (P-gp, ABCB1) confer multidrug resistance and mutant ABC proteins are responsible for many protein-folding diseases such as cystic fibrosis. Identification of the tariquidar-binding site has been the subject of intensive molecular modeling studies because it is the most potent inhibitor and corrector of P-gp. Tariquidar is a unique P-gp inhibitor because it locks the pump in a conformation that blocks drug efflux but activates ATPase activity. In silico docking studies have identified several potential tariquidar-binding sites. Here, we show through cross-linking studies that tariquidar most likely binds to sites within the transmembrane (TM) segments located in one wing or at the interface between the two wings (12 TM segments form 2 divergent wings). We then introduced arginine residues at all positions in the 12 TM segments (223 mutants) of P-gp. The rationale was that a charged residue in the drug-binding pocket would disrupt hydrophobic interaction with tariquidar and inhibit its ability to rescue processing mutants or stimulate ATPase activity. Arginines introduced at 30 positions significantly inhibited tariquidar rescue of a processing mutant and activation of ATPase activity. The results suggest that tariquidar binds to a site within the drug-binding pocket at the interface between the TM segments of both structural wings. Tariquidar differed from other drug substrates, however, as it stabilized the first TM domain. Stabilization of the first TM domain appears to be a key mechanism for high efficiency rescue of ABC processing mutants that cause disease.
Keywords: ABC transporter; membrane enzyme; membrane protein; protein cross-linking; protein folding.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Cysteines introduced into extracellular loops 1 and 4 of human P-glycoprotein that are close only in the open conformation spontaneously form a disulfide bond that inhibits drug efflux and ATPase activity.J Biol Chem. 2014 Sep 5;289(36):24749-58. doi: 10.1074/jbc.M114.583021. Epub 2014 Jul 22. J Biol Chem. 2014. PMID: 25053414 Free PMC article.
-
Tariquidar inhibits P-glycoprotein drug efflux but activates ATPase activity by blocking transition to an open conformation.Biochem Pharmacol. 2014 Dec 15;92(4):558-66. doi: 10.1016/j.bcp.2014.10.006. Epub 2014 Oct 22. Biochem Pharmacol. 2014. PMID: 25456855
-
Thiol-reactive drug substrates of human P-glycoprotein label the same sites to activate ATPase activity in membranes or dodecyl maltoside detergent micelles.Biochem Biophys Res Commun. 2017 Jul 8;488(4):573-577. doi: 10.1016/j.bbrc.2017.05.106. Epub 2017 May 19. Biochem Biophys Res Commun. 2017. PMID: 28533092
-
Determining the structure and mechanism of the human multidrug resistance P-glycoprotein using cysteine-scanning mutagenesis and thiol-modification techniques.Biochim Biophys Acta. 1999 Dec 6;1461(2):315-25. doi: 10.1016/s0005-2736(99)00165-0. Biochim Biophys Acta. 1999. PMID: 10581364 Review.
-
Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor.Expert Rev Anticancer Ther. 2007 Apr;7(4):447-59. doi: 10.1586/14737140.7.4.447. Expert Rev Anticancer Ther. 2007. PMID: 17428165 Review.
Cited by
-
Mutational analysis reveals the importance of residues of the access tunnel inhibitor site to human P-glycoprotein (ABCB1)-mediated transport.Protein Sci. 2024 Sep;33(9):e5155. doi: 10.1002/pro.5155. Protein Sci. 2024. PMID: 39194126
-
Comparison of the Blood-Brain Barrier Transport and Vulnerability to P-Glycoprotein-Mediated Drug-Drug Interaction of Domperidone versus Metoclopramide Assessed Using In Vitro Assay and PET Imaging.Pharmaceutics. 2022 Aug 9;14(8):1658. doi: 10.3390/pharmaceutics14081658. Pharmaceutics. 2022. PMID: 36015284 Free PMC article.
-
Insights into P-Glycoprotein Inhibitors: New Inducers of Immunogenic Cell Death.Cells. 2020 Apr 22;9(4):1033. doi: 10.3390/cells9041033. Cells. 2020. PMID: 32331368 Free PMC article.
-
Computational Insights into Allosteric Conformational Modulation of P-Glycoprotein by Substrate and Inhibitor Binding.Molecules. 2020 Dec 18;25(24):6006. doi: 10.3390/molecules25246006. Molecules. 2020. PMID: 33353070 Free PMC article.
-
Lysine 268 adjacent to transmembrane helix 5 of hamster P-glycoprotein is the major photobinding site of iodomycin in CHO B30 cells.FEBS Open Bio. 2021 Apr;11(4):1084-1092. doi: 10.1002/2211-5463.13112. Epub 2021 Feb 28. FEBS Open Bio. 2021. PMID: 33565718 Free PMC article.
References
-
- Borst P., and Elferink R. O. (2002) Mammalian abc transporters in health and disease. Annu. Rev. Biochem. 71, 537–592 - PubMed
-
- Loo T. W., and Clarke D. M. (2014) Tariquidar inhibits P-glycoprotein drug efflux but activates ATPase activity by blocking transition to an open conformation. Biochem. Pharmacol. 92, 558–566 - PubMed
-
- Wagner C. C., Bauer M., Karch R., Feurstein T., Kopp S., Chiba P., Kletter K., Löscher W., Müller M., Zeitlinger M., and Langer O. (2009) A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood-brain barrier with (R)-11C-verapamil and PET. J. Nucl. Med. 50, 1954–1961 - PMC - PubMed
-
- Kannan P., Telu S., Shukla S., Ambudkar S.V., Pike V.W., Halldin C., Gottesman M.M., Innis R.B., and Hall M.D. (2011) The “specific” P-glycoprotein inhibitor tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2). ACS Chem. Neurosci. 2, 82–89 - PMC - PubMed
-
- Loo T. W., and Clarke D. M. (1997) Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J. Biol. Chem. 272, 709–712 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

