Retinoblastoma-binding Protein 4-regulated Classical Nuclear Transport Is Involved in Cellular Senescence
- PMID: 26491019
- PMCID: PMC4705941
- DOI: 10.1074/jbc.M115.681908
Retinoblastoma-binding Protein 4-regulated Classical Nuclear Transport Is Involved in Cellular Senescence
Abstract
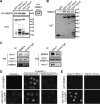
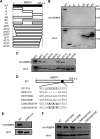
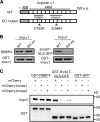
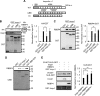
Nucleocytoplasmic trafficking is a fundamental cellular process in eukaryotic cells. Here, we demonstrated that retinoblastoma-binding protein 4 (RBBP4) functions as a novel regulatory factor to increase the efficiency of importin α/β-mediated nuclear import. RBBP4 accelerates the release of importin β1 from importin α via competitive binding to the importin β-binding domain of importin α in the presence of RanGTP. Therefore, it facilitates importin α/β-mediated nuclear import. We showed that the importin α/β pathway is down-regulated in replicative senescent cells, concomitant with a decrease in RBBP4 level. Knockdown of RBBP4 caused both suppression of nuclear transport and induction of cellular senescence. This is the first report to identify a factor that competes with importin β1 to bind to importin α, and it demonstrates that the loss of this factor can trigger cellular senescence.
Keywords: IBB; cellular regulation; cellular senescence; importin α; importin β1; nuclear transport; protein-protein interaction; structural model.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


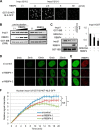
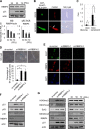
Similar articles
-
Thioredoxin-related transmembrane protein 2 (TMX2) regulates the Ran protein gradient and importin-β-dependent nuclear cargo transport.Sci Rep. 2019 Oct 25;9(1):15296. doi: 10.1038/s41598-019-51773-x. Sci Rep. 2019. PMID: 31653923 Free PMC article.
-
Npap60/Nup50 is a tri-stable switch that stimulates importin-alpha:beta-mediated nuclear protein import.Cell. 2002 Aug 9;110(3):349-60. doi: 10.1016/s0092-8674(02)00836-x. Cell. 2002. PMID: 12176322
-
Dynamics of the STAT3 transcription factor: nuclear import dependent on Ran and importin-β1.PLoS One. 2011;6(5):e20188. doi: 10.1371/journal.pone.0020188. Epub 2011 May 19. PLoS One. 2011. PMID: 21625522 Free PMC article.
-
The importin β binding domain as a master regulator of nucleocytoplasmic transport.Biochim Biophys Acta. 2011 Sep;1813(9):1578-92. doi: 10.1016/j.bbamcr.2010.10.012. Epub 2010 Oct 26. Biochim Biophys Acta. 2011. PMID: 21029753 Free PMC article. Review.
-
Nucleocytoplasmic protein transport and recycling of Ran.Cell Struct Funct. 1999 Dec;24(6):425-33. doi: 10.1247/csf.24.425. Cell Struct Funct. 1999. PMID: 10698256 Review.
Cited by
-
Importin-7-dependent nuclear translocation of the Flavivirus core protein is required for infectious virus production.PLoS Pathog. 2024 Aug 15;20(8):e1012409. doi: 10.1371/journal.ppat.1012409. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39146232 Free PMC article.
-
ATP-dependent chromatin remodelers in ageing and age-related disorders.Biogerontology. 2021 Feb;22(1):1-17. doi: 10.1007/s10522-020-09899-3. Epub 2020 Sep 23. Biogerontology. 2021. PMID: 32968929 Review.
-
The conserved histone chaperone LIN-53 is required for normal lifespan and maintenance of muscle integrity in Caenorhabditis elegans.Aging Cell. 2019 Dec;18(6):e13012. doi: 10.1111/acel.13012. Epub 2019 Aug 9. Aging Cell. 2019. PMID: 31397537 Free PMC article.
-
Characterization of the nuclear import of the human CHD4-NuRD complex.J Cell Sci. 2023 Apr 1;136(7):jcs260724. doi: 10.1242/jcs.260724. Epub 2023 Apr 6. J Cell Sci. 2023. PMID: 36861403 Free PMC article.
-
Loss of RBBP4 results in defective inner cell mass, severe apoptosis, hyperacetylated histones and preimplantation lethality in mice†.Biol Reprod. 2020 Jun 23;103(1):13-23. doi: 10.1093/biolre/ioaa046. Biol Reprod. 2020. PMID: 32285100 Free PMC article.
References
-
- Terry L. J., Shows E. B., and Wente S. R. (2007) Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 318, 1412–1416 - PubMed
-
- Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., and Adam S. A. (2004) Importin α: a multipurpose nuclear-transport receptor. Trends Cell Biol. 14, 505–514 - PubMed
-
- Miyamoto Y., Boag P. R., Hime G. R., and Loveland K. L. (2012) Regulated nucleocytoplasmic transport during gametogenesis. Biochim. Biophys. Acta 1819, 616–630 - PubMed
-
- Lee S. J., Matsuura Y., Liu S. M., and Stewart M. (2005) Structural basis for nuclear import complex dissociation by RanGTP. Nature 435, 693–696 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

