CARTS biogenesis requires VAP-lipid transfer protein complexes functioning at the endoplasmic reticulum-Golgi interface
- PMID: 26490117
- PMCID: PMC4678024
- DOI: 10.1091/mbc.E15-08-0599
CARTS biogenesis requires VAP-lipid transfer protein complexes functioning at the endoplasmic reticulum-Golgi interface
Abstract
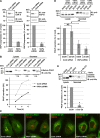
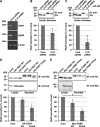
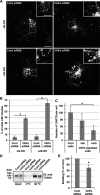
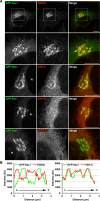
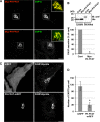
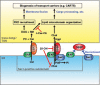
Vesicle-associated membrane protein-associated protein (VAP) is an endoplasmic reticulum (ER)-resident integral membrane protein that controls a nonvesicular mode of ceramide and cholesterol transfer from the ER to the Golgi complex by interacting with ceramide transfer protein and oxysterol-binding protein (OSBP), respectively. We report that VAP and its interacting proteins are required for the processing and secretion of pancreatic adenocarcinoma up-regulated factor, whose transport from the trans-Golgi network (TGN) to the cell surface is mediated by transport carriers called "carriers of the trans-Golgi network to the cell surface" (CARTS). In VAP-depleted cells, diacylglycerol level at the TGN was decreased and CARTS formation was impaired. We found that VAP forms a complex with not only OSBP but also Sac1 phosphoinositide phosphatase at specialized ER subdomains that are closely apposed to the trans-Golgi/TGN, most likely reflecting membrane contact sites. Immobilization of ER-Golgi contacts dramatically reduced CARTS production, indicating that association-dissociation dynamics of the two membranes are important. On the basis of these findings, we propose that the ER-Golgi contacts play a pivotal role in lipid metabolism to control the biogenesis of transport carriers from the TGN.
© 2015 Wakana et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
Similar articles
-
The ER cholesterol sensor SCAP promotes CARTS biogenesis at ER-Golgi membrane contact sites.J Cell Biol. 2021 Jan 4;220(1):e202002150. doi: 10.1083/jcb.202002150. J Cell Biol. 2021. PMID: 33156328 Free PMC article.
-
Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein.Mol Biol Cell. 2006 Jun;17(6):2604-16. doi: 10.1091/mbc.e06-01-0060. Epub 2006 Mar 29. Mol Biol Cell. 2006. PMID: 16571669 Free PMC article.
-
Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport.Mol Biol Cell. 2008 Sep;19(9):3871-84. doi: 10.1091/mbc.e08-05-0498. Epub 2008 Jul 9. Mol Biol Cell. 2008. PMID: 18614794 Free PMC article.
-
CERT and intracellular trafficking of ceramide.Biochim Biophys Acta. 2007 Jun;1771(6):644-53. doi: 10.1016/j.bbalip.2007.01.009. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17314061 Review.
-
Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites.FEBS Lett. 2019 Sep;593(17):2366-2377. doi: 10.1002/1873-3468.13511. Epub 2019 Jul 8. FEBS Lett. 2019. PMID: 31254361 Review.
Cited by
-
The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers.Cells. 2021 Jun 28;10(7):1618. doi: 10.3390/cells10071618. Cells. 2021. PMID: 34203456 Free PMC article. Review.
-
The Link between VAPB Loss of Function and Amyotrophic Lateral Sclerosis.Cells. 2021 Jul 23;10(8):1865. doi: 10.3390/cells10081865. Cells. 2021. PMID: 34440634 Free PMC article. Review.
-
ER as master regulator of membrane trafficking and organelle function.J Cell Biol. 2022 Oct 3;221(10):e202205135. doi: 10.1083/jcb.202205135. Epub 2022 Sep 15. J Cell Biol. 2022. PMID: 36108241 Free PMC article. Review.
-
Bridging the molecular and biological functions of the oxysterol-binding protein family.Cell Mol Life Sci. 2018 Sep;75(17):3079-3098. doi: 10.1007/s00018-018-2795-y. Epub 2018 Mar 13. Cell Mol Life Sci. 2018. PMID: 29536114 Free PMC article. Review.
-
Sending out molecules from the TGN.Curr Opin Cell Biol. 2021 Aug;71:55-62. doi: 10.1016/j.ceb.2021.02.005. Epub 2021 Mar 8. Curr Opin Cell Biol. 2021. PMID: 33706234 Free PMC article. Review.
References
-
- Alpy F, Rousseau A, Schwab Y, Legueux F, Stoll I, Wendling C, Spiegelhalter C, Kessler P, Mathelin C, Rio MC, et al. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J Cell Sci. 2013;126:5500–5512. - PubMed
-
- Balch WE, Glick BS, Rothman JE. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984;39:525–536. - PubMed
-
- Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

