New Insights into the RNA-Binding and E3 Ubiquitin Ligase Activities of Roquins
- PMID: 26489670
- PMCID: PMC4614863
- DOI: 10.1038/srep15660
New Insights into the RNA-Binding and E3 Ubiquitin Ligase Activities of Roquins
Abstract
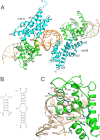
Roquins are a family of highly conserved RNA-binding proteins that also contain a RING-type E3 ubiquitin ligase domain. They repress constitutive decay elements containing mRNAs and play a critical role in RNA homeostasis and immunological self-tolerance. Here we present the crystal structures of the RNA-binding region of Roquin paralog RC3H2 in both apo- and RNA-bound forms. The RNA-binding region has a bipartite architecture composed of ROQ and HEPN domains, and can bind to stem-loop and double-stranded RNAs simultaneously. The two domains undergo a large orientation change to accommodate RNA duplex binding. We profiled E2 ubiquitin-conjugating enzymes that pair with Roquins and found that RC3H1 and RC3H2 interact with two sets of overlapping but not identical E2 enzymes to drive the assembly of polyubiquitin chains of different linkages. Crystal structures, small-angle X-ray scattering, and E2 profiling revealed that while the two paralogs are highly homologous, RC3H2 and RC3H1 are different in their structures and functions. We also demonstrated that RNA duplex binding to RC3H2 cross-talks with its E3 ubiquitin ligase function using an in vitro auto-ubiquitination assay.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Structure of human Roquin-2 and its complex with constitutive-decay element RNA.Acta Crystallogr F Struct Biol Commun. 2015 Aug;71(Pt 8):1048-54. doi: 10.1107/S2053230X15011887. Epub 2015 Jul 29. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26249698 Free PMC article.
-
The human RNA-binding protein and E3 ligase MEX-3C binds the MEX-3-recognition element (MRE) motif with high affinity.J Biol Chem. 2017 Sep 29;292(39):16221-16234. doi: 10.1074/jbc.M117.797746. Epub 2017 Aug 14. J Biol Chem. 2017. PMID: 28808060 Free PMC article.
-
The ROQ domain of Roquin recognizes mRNA constitutive-decay element and double-stranded RNA.Nat Struct Mol Biol. 2014 Aug;21(8):679-85. doi: 10.1038/nsmb.2857. Epub 2014 Jul 13. Nat Struct Mol Biol. 2014. PMID: 25026078 Free PMC article.
-
TRIM-NHL as RNA Binding Ubiquitin E3 Ligase (RBUL): Implication in development and disease pathogenesis.Biochim Biophys Acta Mol Basis Dis. 2021 Jul 1;1867(7):166066. doi: 10.1016/j.bbadis.2020.166066. Epub 2021 Jan 6. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33418035 Review.
-
Emerging RNA-binding roles in the TRIM family of ubiquitin ligases.Biol Chem. 2019 Oct 25;400(11):1443-1464. doi: 10.1515/hsz-2019-0158. Biol Chem. 2019. PMID: 31120853 Review.
Cited by
-
Ubiquitin signaling in immune responses.Cell Res. 2016 Apr;26(4):457-83. doi: 10.1038/cr.2016.40. Epub 2016 Mar 25. Cell Res. 2016. PMID: 27012466 Free PMC article. Review.
-
MALT1 substrate cleavage: what is it good for?Front Immunol. 2024 May 28;15:1412347. doi: 10.3389/fimmu.2024.1412347. eCollection 2024. Front Immunol. 2024. PMID: 38863711 Free PMC article. Review.
-
RNA-Binding RING E3-Ligase DZIP3/hRUL138 Stabilizes Cyclin D1 to Drive Cell-Cycle and Cancer Progression.Cancer Res. 2021 Jan 15;81(2):315-331. doi: 10.1158/0008-5472.CAN-20-1871. Epub 2020 Oct 16. Cancer Res. 2021. PMID: 33067265 Free PMC article.
-
Identification of new high affinity targets for Roquin based on structural conservation.Nucleic Acids Res. 2018 Dec 14;46(22):12109-12125. doi: 10.1093/nar/gky908. Nucleic Acids Res. 2018. PMID: 30295819 Free PMC article.
-
Functional insights from a surface antigen mRNA-bound proteome.Elife. 2021 Mar 30;10:e68136. doi: 10.7554/eLife.68136. Elife. 2021. PMID: 33783358 Free PMC article.
References
-
- Castello A., Fischer B., Hentze M. W. & Preiss T. RNA-binding proteins in Mendelian disease. Trends Genet. 29, 318–327 (2013). - PubMed
-
- Lenzken S. C., Achsel T., Carrì M. T. & Barabino S. M. L. Neuronal RNA-binding proteins in health and disease. Wiley Interdiscip. Rev. RNA 5, 565–576 (2014). - PubMed
-
- Heissmeyer V. & Vogel K. U. Molecular control of Tfh-cell differentiation by Roquin family proteins. Immunol. Rev. 253, 273–89 (2013). - PubMed
-
- Vinuesa C. G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

