Myocardial Delivery of Lipidoid Nanoparticle Carrying modRNA Induces Rapid and Transient Expression
- PMID: 26471463
- PMCID: PMC4754552
- DOI: 10.1038/mt.2015.193
Myocardial Delivery of Lipidoid Nanoparticle Carrying modRNA Induces Rapid and Transient Expression
Abstract
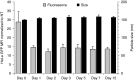
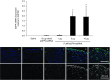
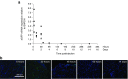
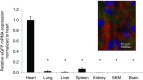
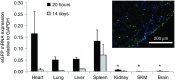
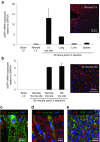
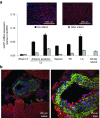
Nanoparticle-based delivery of nucleotides offers an alternative to viral vectors for gene therapy. We report highly efficient in vivo delivery of modified mRNA (modRNA) to rat and pig myocardium using formulated lipidoid nanoparticles (FLNP). Direct myocardial injection of FLNP containing 1-10 μg eGFPmodRNA in the rat (n = 3 per group) showed dose-dependent enhanced green fluorescent protein (eGFP) mRNA levels in heart tissue 20 hours after injection, over 60-fold higher than for naked modRNA. Off-target expression, including lung, liver, and spleen, was <10% of that in heart. Expression kinetics after injecting 5 μg FLNP/eGFPmodRNA showed robust expression at 6 hours that reduced by half at 48 hours and was barely detectable at 2 weeks. Intracoronary administration of 10 μg FLNP/eGFPmodRNA also proved successful, although cardiac expression of eGFP mRNA at 20 hours was lower than direct injection, and off-target expression was correspondingly higher. Findings were confirmed in a pilot study in pigs using direct myocardial injection as well as percutaneous intracoronary delivery, in healthy and myocardial infarction models, achieving expression throughout the ventricular wall. Fluorescence microscopy revealed GFP-positive cardiomyocytes in treated hearts. This nanoparticle-enabled approach for highly efficient, rapid and short-term mRNA expression in the heart offers new opportunities to optimize gene therapies for enhancing cardiac function and regeneration.
Figures
Similar articles
-
Lipidoid mRNA Nanoparticles for Myocardial Delivery in Rodents.Methods Mol Biol. 2017;1521:153-166. doi: 10.1007/978-1-4939-6588-5_10. Methods Mol Biol. 2017. PMID: 27910047 Free PMC article.
-
Synthetic chemically modified mrna-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction.Mol Pharm. 2015 Mar 2;12(3):991-6. doi: 10.1021/mp5006239. Epub 2015 Jan 27. Mol Pharm. 2015. PMID: 25588055
-
Delivery of Modified mRNA in a Myocardial Infarction Mouse Model.J Vis Exp. 2020 Jun 11;(160). doi: 10.3791/60832. J Vis Exp. 2020. PMID: 32597835
-
Novel polymer carriers and gene constructs for treatment of myocardial ischemia and infarction.J Control Release. 2008 Dec 18;132(3):260-6. doi: 10.1016/j.jconrel.2008.06.024. Epub 2008 Jul 6. J Control Release. 2008. PMID: 18662730 Free PMC article. Review.
-
Modified mRNA as a Treatment for Myocardial Infarction.Int J Mol Sci. 2023 Mar 1;24(5):4737. doi: 10.3390/ijms24054737. Int J Mol Sci. 2023. PMID: 36902165 Free PMC article. Review.
Cited by
-
Ablation of a Single N-Glycosylation Site in Human FSTL 1 Induces Cardiomyocyte Proliferation and Cardiac Regeneration.Mol Ther Nucleic Acids. 2018 Dec 7;13:133-143. doi: 10.1016/j.omtn.2018.08.021. Epub 2018 Sep 1. Mol Ther Nucleic Acids. 2018. PMID: 30290305 Free PMC article.
-
Notch Intracellular Domain Plasmid Delivery via Poly(Lactic-Co-Glycolic Acid) Nanoparticles to Upregulate Notch Pathway Molecules.Front Cardiovasc Med. 2021 Sep 28;8:707897. doi: 10.3389/fcvm.2021.707897. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34651022 Free PMC article.
-
Endovascular transplantation of mRNA-enhanced mesenchymal stromal cells results in superior therapeutic protein expression in swine heart.Mol Ther Methods Clin Dev. 2024 Feb 27;32(2):101225. doi: 10.1016/j.omtm.2024.101225. eCollection 2024 Jun 13. Mol Ther Methods Clin Dev. 2024. PMID: 38516693 Free PMC article.
-
Structurally Programmed Assembly of Translation Initiation Nanoplex for Superior mRNA Delivery.ACS Nano. 2017 Mar 28;11(3):2531-2544. doi: 10.1021/acsnano.6b08447. Epub 2017 Feb 14. ACS Nano. 2017. PMID: 28157292 Free PMC article.
-
Modified mRNAs in the Cardiovascular System: A New Platform for Gene Therapy.Mol Ther. 2017 Jun 7;25(6):1266-1268. doi: 10.1016/j.ymthe.2017.05.011. Epub 2017 May 24. Mol Ther. 2017. PMID: 28550973 Free PMC article. No abstract available.
References
-
- Kawase, Y, Ly, HQ, Prunier, F, Lebeche, D, Shi, Y, Jin, H et al. (2008). Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol 51: 1112–1119. - PubMed
-
- Zsebo, K, Yaroshinsky, A, Rudy, JJ, Wagner, K, Greenberg, B, Jessup, M et al. (2014). Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res 114: 101–108. - PubMed
-
- Hayward, C, Banner, NR, Morley-Smith, A, Lyon, AR and Harding, SE (2015). The Current and Future Landscape of SERCA Gene Therapy for Heart Failure: A Clinical Perspective. Hum Gene Ther 26: 293–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

